J Korean Med Sci.
2022 Feb;37(7):e52. 10.3346/jkms.2022.37.e52.
Case Reports of Acute Transverse Myelitis Associated With mRNA Vaccine for COVID-19
- Affiliations
-
- 1Department of Neurology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Neurology, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2526515
- DOI: http://doi.org/10.3346/jkms.2022.37.e52
Abstract
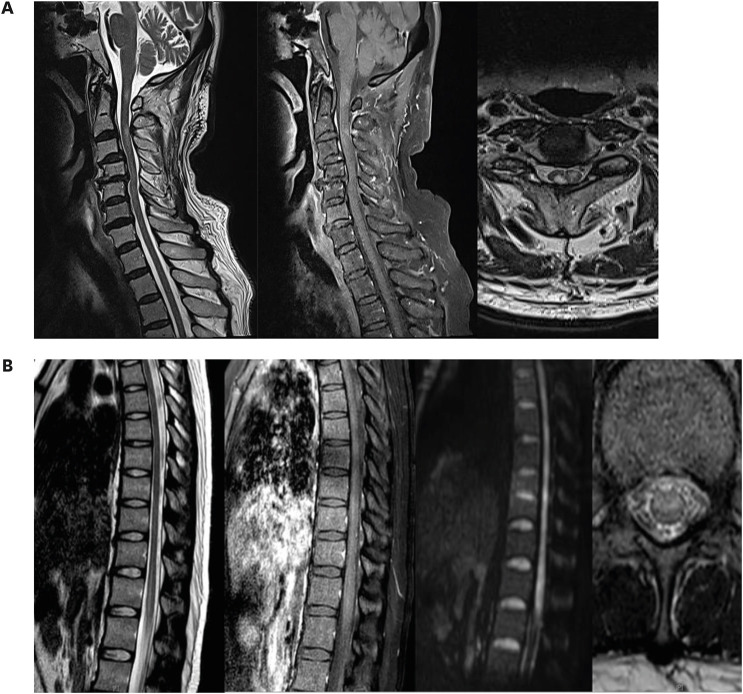
- Acute transverse myelitis (ATM) has been reported as rare complication of vaccination. Herein, we report 2 cases of ATM after the administration of an mRNA vaccine for coronavirus disease 2019 (COVID-19). The first one is an 81-year-old man who received the BNT162b2 vaccine. He presented with bilateral hand weakness. Spine magnetic resonance imaging (MRI) showed high signal intensity from the C1 to C3 vertebrae. The second is a 23-year-old woman who received the BNT162b2 vaccine and experienced tingling in her legs. Spine MRI showed a high signal intensity lesion at the conus medullaris. These patients were treated with intravenous methylprednisolone and their symptoms improved slightly. Careful follow-up is needed to identify adverse events after the administration of mRNA vaccines for COVID-19.
Figure
Reference
-
1. Vitiello A, Ferrara F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacology. 2021; 29(3):645–649. PMID: 33932192.
Article2. Alimehmeti I. Efficacy and safety of AZD1222, BNT162b2 and mRNA-1273 vaccines against SARS-CoV-2. Albanian J Trauma Emerg Surg. 2021; 5(1):791–796.
Article3. DeStefano F, Verstraeten T, Jackson LA, Okoro CA, Benson P, Black SB, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol. 2003; 60(4):504–509. PMID: 12707063.
Article4. Pagenkopf C, Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against COVID-19. J Neuroimmunol. 2021; 358:577606. PMID: 34182207.
Article5. Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222). Front Immunol. 2021; 12:653786. PMID: 33981305.
Article6. Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021; 106:376–381. PMID: 33866000.
Article7. Agmon-Levin N, Kivity S, Szyper-Kravitz M, Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009; 18(13):1198–1204. PMID: 19880568.
Article8. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020; 217:108480. PMID: 32461193.
Article9. Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-10 mRNA vaccination. JAMA. 2021; 326(14):1390–1399. PMID: 34477808.
Article10. Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021; 89(5):856–857. PMID: 33710649.11. Khayat-Khoei M, Bhattacharyya S, Katz J, Harrison D, Tauhid S, Bruso P, et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2021; 1–14.
Article12. Fitzsimmons W, Nance CS. Sudden onset of myelitis after COVID-19 vaccination: an under-recognized severe rare adverse event. SSRN. May. 5. 2021; https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3841558 .
Article13. Gao JJ, Tseng HP, Lin CL, Shiu JS, Lee MH, Liu CH. Acute transverse myelitis following COVID-19 vaccination. Vaccines (Basel). 2021; 9(9):1008. PMID: 34579245.
Article14. Korea Disease Center Agency. Updated 2021. Accessed August 10, 2021. http://kdca.go.kr/board/board.es?mid=a20501000000&bid=0015&list_no=716441&act=view .15. Lee MA, Lee C, Park JH, Lee JH. Early-onset myasthenia gravis following COVID-19 vaccination. J Korean Med Sci. Forthcoming 2022. DOI: 10.3346/jkms.2022.37.e50.
Article16. Kim JW, Kim YG, Park YC, Choi S, Lee S, Min HJ, et al. Guillain-Barre syndrome after two COVID-19 vaccinations: two case reports with follow-up electrodiagnostic study. J Korean Med Sci. Forthcoming 2022. DOI: 10.3346/jkms.2022.37.e58.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- mRNA COVID-19 Vaccine-Associated Subserosal Eosinophilic Gastroenteritis: A Case Report
- A Case of Acute Transverse Myelitis with Hepatitis B Virus Infection
- Herpes zoster ophthalmicus after COVID-19 vaccine booster in healthy younger adult: a case report
- Hypertensive crisis following mRNA COVID-19 vaccination in adolescents: two case reports
- Two Cases of Cataract after COVID-19 mRNA Vaccine Injection