Endocrinol Metab.
2021 Dec;36(6):1287-1297. 10.3803/EnM.2021.1217.
Clinical and Molecular Characteristics of PRKACA L206R Mutant Cortisol-Producing Adenomas in Korean Patients
- Affiliations
-
- 1Translational Research Institute, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- 2Department of Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 4Division of Surgery, Thyroid Center, Seoul National University Cancer Hospital, Seoul, Korea
- 5Department of Surgery, Chung-Ang University Hospital, Seoul, Korea
- 6Department of Laboratory Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 7Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2523497
- DOI: http://doi.org/10.3803/EnM.2021.1217
Abstract
- Background
An activating mutation (c.617A>C/p.Lys206Arg, L206R) in protein kinase cAMP-activated catalytic subunit alpha (PRKACA) has been reported in 35% to 65% of cases of cortisol-producing adenomas (CPAs). We aimed to compare the clinical characteristics and transcriptome analysis between PRKACA L206R mutants and wild-type CPAs in Korea.
Methods
We included 57 subjects with CPAs who underwent adrenalectomy at Seoul National University Hospital. Sanger sequencing for PRKACA was conducted in 57 CPA tumor tissues. RNA sequencing was performed in 13 fresh-frozen tumor tissues.
Results
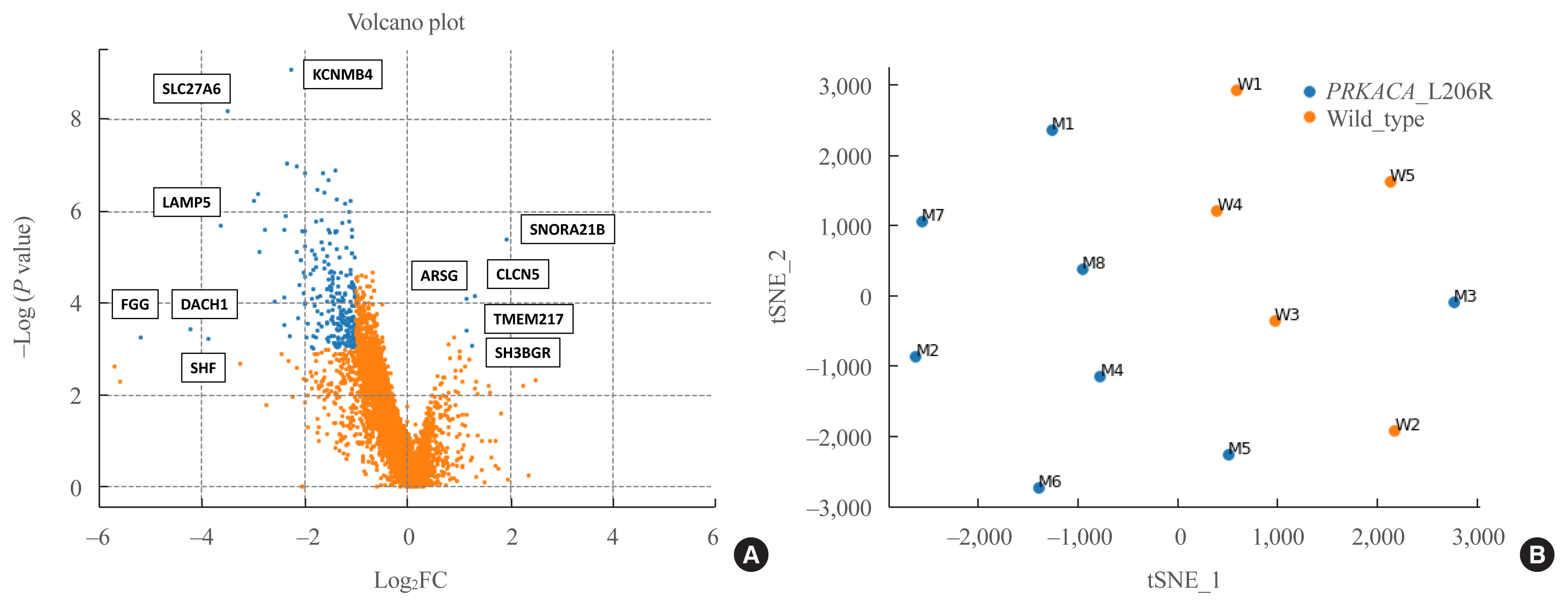
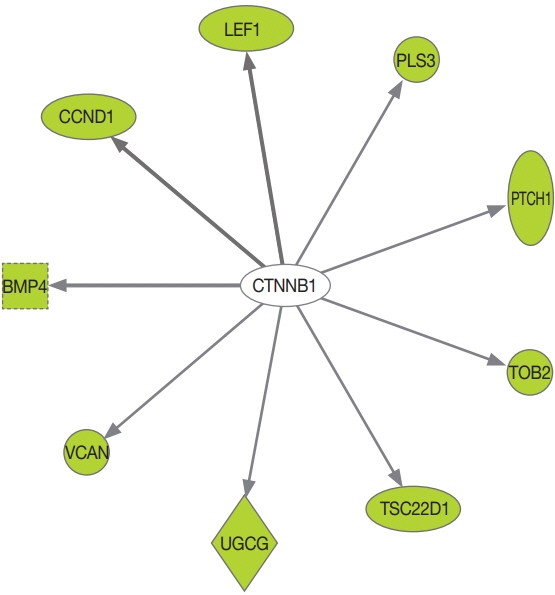
The prevalence of the PRKACA L206R mutation was 51% (29/57). The mean age of the study subjects was 42±12 years, and 87.7% (50/57) of the patients were female. Subjects with PRKACA L206R mutant CPAs showed smaller adenoma size (3.3±0.7 cm vs. 3.8±1.2 cm, P=0.059) and lower dehydroepiandrosterone sulfate levels (218±180 ng/mL vs. 1,511±3,307 ng/mL, P=0.001) than those with PRKACA wild-type CPAs. Transcriptome profiling identified 244 differentially expressed genes (DEGs) between PRKACA L206R mutant (n=8) and wild-type CPAs (n=5), including five upregulated and 239 downregulated genes in PRKACA L206R mutant CPAs (|fold change| ≥2, P<0.05). Among the upstream regulators of DEGs, CTNNB1 was the most significant transcription regulator. In several pathway analyses, the Wnt signaling pathway was downregulated and the steroid biosynthesis pathway was upregulated in PRKACA mutants. Protein-protein interaction analysis also showed that PRKACA downregulates Wnt signaling and upregulates steroid biosynthesis.
Conclusion
The PRKACA L206R mutation in CPAs causes high hormonal activity with a limited proliferative capacity, as supported by transcriptome profiling.
Keyword
Figure
Reference
-
1. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015; 386:913–27.
Article2. Lodish M, Stratakis CA. A genetic and molecular update on adrenocortical causes of Cushing syndrome. Nat Rev Endocrinol. 2016; 12:255–62.
Article3. Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000; 26:89–92.
Article4. Libe R, Horvath A, Vezzosi D, Fratticci A, Coste J, Perlemoine K, et al. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab. 2011; 96:E208–14.5. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991; 325:1688–95.
Article6. Beuschlein F, Fassnacht M, Assie G, Calebiro D, Stratakis CA, Osswald A, et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med. 2014; 370:1019–28.
Article7. Sato Y, Maekawa S, Ishii R, Sanada M, Morikawa T, Shiraishi Y, et al. Recurrent somatic mutations underlie corticotropin-independent Cushing’s syndrome. Science. 2014; 344:917–20.
Article8. Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science. 2014; 344:913–7.
Article9. Goh G, Scholl UI, Healy JM, Choi M, Prasad ML, Nelson-Williams C, et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat Genet. 2014; 46:613–7.
Article10. Dalmazi GD, Beuschlein F. PRKACA mutations in adrenal adenomas: genotype/phenotype correlations. Horm Metab Res. 2017; 49:301–6.
Article11. Calebiro D, Hannawacker A, Lyga S, Bathon K, Zabel U, Ronchi C, et al. PKA catalytic subunit mutations in adrenocortical Cushing’s adenoma impair association with the regulatory subunit. Nat Commun. 2014; 5:5680.
Article12. Thiel A, Reis AC, Haase M, Goh G, Schott M, Willenberg HS, et al. PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases. Eur J Endocrinol. 2015; 172:677–85.
Article13. Zennaro MC, Boulkroun S, Fernandes-Rosa F. Genetic causes of functional adrenocortical adenomas. Endocr Rev. 2017; 38:516–37.
Article14. Nakajima Y, Okamura T, Gohko T, Satoh T, Hashimoto K, Shibusawa N, et al. Somatic mutations of the catalytic subunit of cyclic AMP-dependent protein kinase (PRKACA) gene in Japanese patients with several adrenal adenomas secreting cortisol [Rapid Communication]. Endocr J. 2014; 61:825–32.
Article15. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014; 30:2114–20.
Article16. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013; 29:15–21.
Article17. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011; 12:323.
Article18. Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016; 44:W90–7.
Article19. Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018; 46:D661–7.
Article20. Kramer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014; 30:523–30.
Article21. Hong AR, Kim JH, Hong ES, Kim IK, Park KS, Ahn CH, et al. Limited diagnostic utility of plasma adrenocorticotropic hormone for differentiation between adrenal Cushing syndrome and Cushing disease. Endocrinol Metab (Seoul). 2015; 30:297–304.
Article22. Dennedy MC, Annamalai AK, Prankerd-Smith O, Freeman N, Vengopal K, Graggaber J, et al. Low DHEAS: a sensitive and specific test for the detection of subclinical hypercortisolism in adrenal incidentalomas. J Clin Endocrinol Metab. 2017; 102:786–92.23. Sugawara T, Saito M, Fujimoto S. Sp1 and SF-1 interact and cooperate in the regulation of human steroidogenic acute regulatory protein gene expression. Endocrinology. 2000; 141:2895–903.
Article24. Zhou W, Wu L, Xie J, Su T, Jiang L, Jiang Y, et al. Steroidogenic acute regulatory protein overexpression correlates with protein kinase A activation in adrenocortical adenoma. PLoS One. 2016; 11:e0162606.
Article25. Di Dalmazi G, Kisker C, Calebiro D, Mannelli M, Canu L, Arnaldi G, et al. Novel somatic mutations in the catalytic subunit of the protein kinase A as a cause of adrenal Cushing’s syndrome: a European multicentric study. J Clin Endocrinol Metab. 2014; 99:E2093–100.
Article26. Almeida MQ, Harran M, Bimpaki EI, Hsiao HP, Horvath A, Cheadle C, et al. Integrated genomic analysis of nodular tissue in macronodular adrenocortical hyperplasia: progression of tumorigenesis in a disorder associated with multiple benign lesions. J Clin Endocrinol Metab. 2011; 96:E728–38.
Article27. Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005; 25:9063–72.
Article28. Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006; 281:9971–6.29. Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, et al. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010; 19:1387–98.
Article30. Gaujoux S, Tissier F, Groussin L, Libe R, Ragazzon B, Launay P, et al. Wnt/beta-catenin and 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab. 2008; 93:4135–40.31. Drelon C, Berthon A, Sahut-Barnola I, Mathieu M, Dumontet T, Rodriguez S, et al. PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun. 2016; 7:12751.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical and Technical Aspects in Free Cortisol Measurement
- CTNNB1 Mutation in Aldosterone Producing Adenoma
- Bilateral Adrenocortical Masses Producing Aldosterone and Cortisol Independently
- A Case of Adrenocortical Carcinoma showing Variable Cortisol Production during the Clinical Course
- Insights into hepatocellular adenomas in Asia: molecular subtypes, clinical characteristics, imaging features, and hepatocellular carcinoma risks