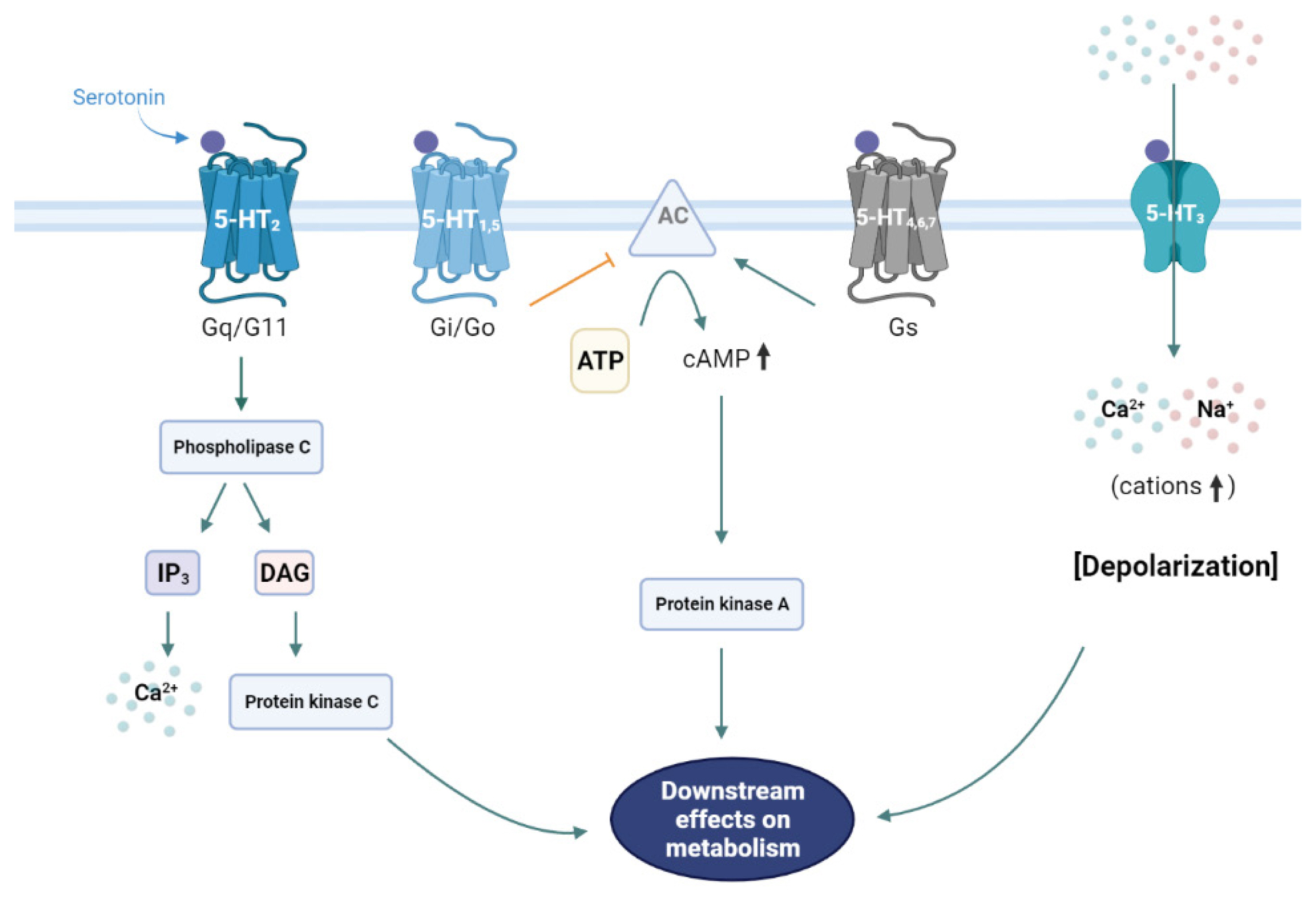
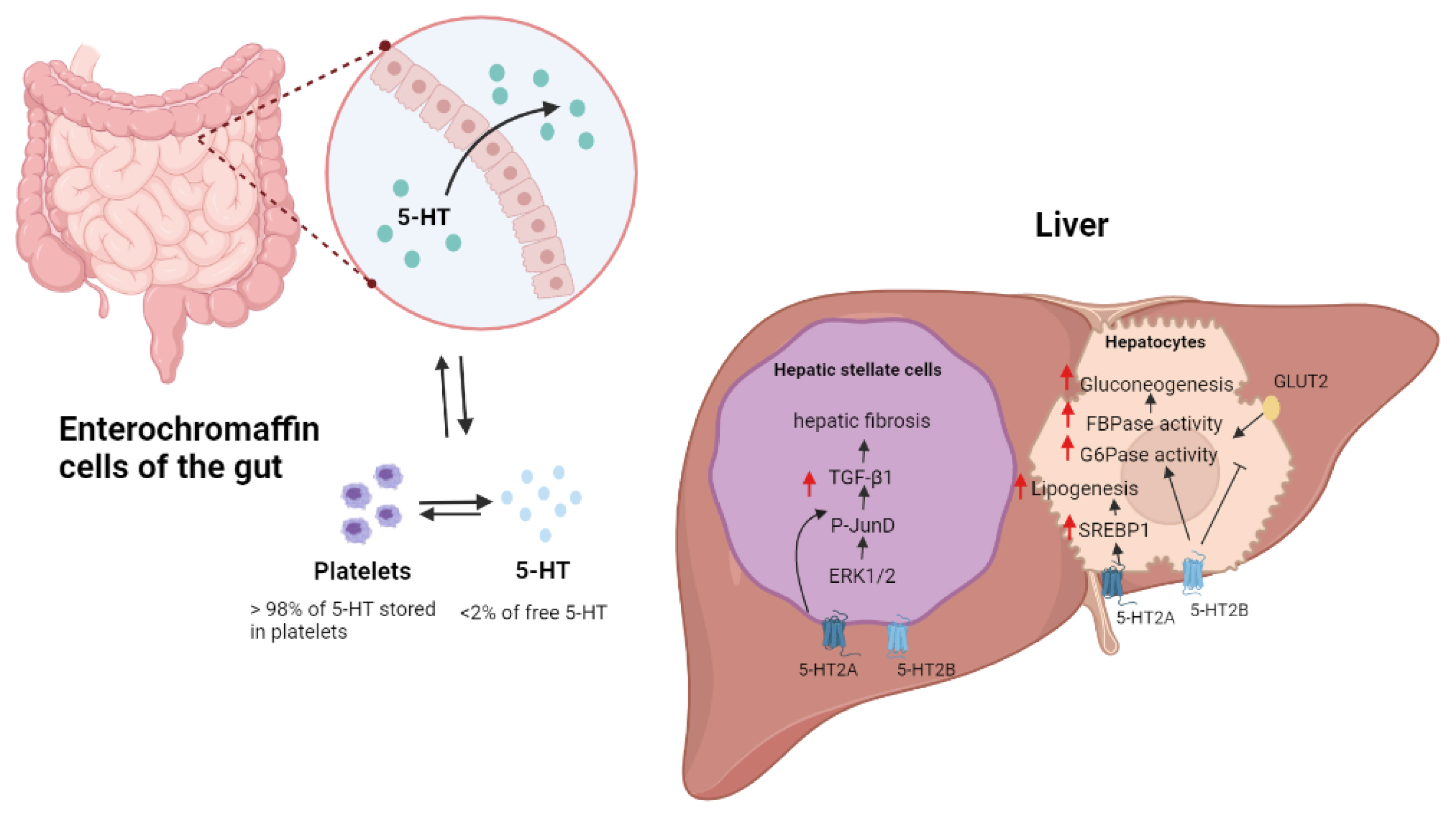
Endocrinol Metab.
2021 Dec;36(6):1151-1160. 10.3803/EnM.2021.1331.
Serotonergic Regulation of Hepatic Energy Metabolism
- Affiliations
-
- 1Department of Biomedical Science and Engineering, Gwangju Institute of Science and Technology, Gwangju, Korea
- 2Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- KMID: 2523484
- DOI: http://doi.org/10.3803/EnM.2021.1331
Abstract
- The liver is a vital organ that regulates systemic energy metabolism and many physiological functions. Nonalcoholic fatty liver disease (NAFLD) is the commonest cause of chronic liver disease and end-stage liver failure. NAFLD is primarily caused by metabolic disruption of lipid and glucose homeostasis. Serotonin (5-hydroxytryptamine [5-HT]) is a biogenic amine with several functions in both the central and peripheral systems. 5-HT functions as a neurotransmitter in the brain and a hormone in peripheral tissues to regulate systemic energy homeostasis. Several recent studies have proposed various roles of 5-HT in hepatic metabolism and inflammation using tissue-specific knockout mice and 5-HT-receptor agonists/antagonists. This review compiles the most recent research on the relationship between 5-HT and hepatic metabolism, and the role of 5-HT signaling as a potential therapeutic target in NAFLD.
Keyword
Figure
Reference
-
1. Abai B. StatPearls. Treasure Island: StatPearls Publishing;2021. Chapter, Physiology, liver. https://www.ncbi.nlm.nih.gov/books/NBK535438 .2. Fotbolcu H, Zorlu E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J Gastroenterol. 2016; 22:4079–90.
Article3. Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021; 17:484–95.
Article4. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015; 62(1 Suppl):S47–64.
Article5. Chao HW, Chao SW, Lin H, Ku HC, Cheng CF. Homeostasis of glucose and lipid in non-alcoholic fatty liver disease. Int J Mol Sci. 2019; 20:298.
Article6. Pei K, Gui T, Kan D, Feng H, Jin Y, Yang Y, et al. An overview of lipid metabolism and nonalcoholic fatty liver disease. Biomed Res Int. 2020; 2020:4020249.
Article7. Oh CM, Park S, Kim H. Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab J. 2016; 40:89–98.
Article8. Yabut JM, Crane JD, Green AE, Keating DJ, Khan WI, Steinberg GR. Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocr Rev. 2019; 40:1092–107.
Article9. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017; 10:1178646917691938.
Article10. Nakamura K, Hasegawa H. Developmental role of tryptophan hydroxylase in the nervous system. Mol Neurobiol. 2007; 35:45–54.
Article11. Prah A, Purg M, Stare J, Vianello R, Mavri J. How monoamine oxidase a decomposes serotonin: an empirical valence bond simulation of the reactive step. J Phys Chem B. 2020; 124:8259–65.
Article12. Gauer F, Craft CM. Circadian regulation of hydroxyindole-O-methyltransferase mRNA levels in rat pineal and retina. Brain Res. 1996; 737:99–109.
Article13. Pissios P, Maratos-Flier E. More than satiety: central serotonin signaling and glucose homeostasis. Cell Metab. 2007; 6:345–7.
Article14. van Galen KA, Ter Horst KW, Serlie MJ. Serotonin, food intake, and obesity. Obes Rev. 2021; 22:e13210.15. Namkung J, Kim H, Park S. Peripheral serotonin: a new player in systemic energy homeostasis. Mol Cells. 2015; 38:1023–8.
Article16. Vandenbussche N, Goadsby PJ. Lorcaserin safety in overweight or obese patients. N Engl J Med. 2019; 380:99.
Article17. Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L. Cancer risk associated with lorcaserin: the FDA’s review of the CAMELLIA-TIMI 61 Trial. N Engl J Med. 2020; 383:1000–2.
Article18. Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003; 460:303–26.
Article19. Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R776–83.
Article20. McGlashon JM, Gorecki MC, Kozlowski AE, Thirnbeck CK, Markan KR, Leslie KL, et al. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metab. 2015; 21:692–705.
Article21. Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013; 123:5061–70.
Article22. Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007; 5:438–49.
Article23. Chandran S, Guo T, Tolliver T, Chen W, Murphy DL, McPherron AC. Effects of serotonin on skeletal muscle growth. BMC Proc. 2012; 6(Suppl 3):O3.
Article24. Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H, et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun. 2015; 6:6794.
Article25. Park H, Oh CM, Park J, Park H, Cui S, Kim HS, et al. Deletion of the serotonin receptor type 3A in mice leads to sudden cardiac death during pregnancy. Circ J. 2015; 79:1807–15.
Article26. Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010; 16:804–8.
Article27. Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011; 31:8998–9009.
Article28. Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf). 2015; 213:561–74.
Article29. Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012; 142:844–54.
Article30. Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009; 29:9683–99.
Article31. Kwon YH, Wang H, Denou E, Ghia JE, Rossi L, Fontes ME, et al. Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell Mol Gastroenterol Hepatol. 2019; 7:709–28.
Article32. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015; 161:264–76.
Article33. Ohara-Imaizumi M, Kim H, Yoshida M, Fujiwara T, Aoyagi K, Toyofuku Y, et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc Natl Acad Sci U S A. 2013; 110:19420–5.
Article34. Kim K, Oh CM, Ohara-Imaizumi M, Park S, Namkung J, Yadav VK, et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology. 2015; 156:444–52.
Article35. Moon JH, Kim YG, Kim K, Osonoi S, Wang S, Saunders DC, et al. Serotonin regulates adult β-cell mass by stimulating perinatal β-cell proliferation. Diabetes. 2020; 69:205–14.
Article36. Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012; 16:588–600.
Article37. Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008; 3:e3301.
Article38. Gutknecht L, Araragi N, Merker S, Waider J, Sommerlandt FM, Mlinar B, et al. Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS One. 2012; 7:e43157.
Article39. Shong KE, Oh CM, Namkung J, Park S, Kim H. Serotonin regulates de novo lipogenesis in adipose tissues through serotonin receptor 2A. Endocrinol Metab (Seoul). 2020; 35:470–9.
Article40. Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015; 21:166–72.
Article41. Omenetti A, Yang L, Gainetdinov RR, Guy CD, Choi SS, Chen W, et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G303–15.
Article42. Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol. 2008; 48:666–75.
Article43. Richardson PD, Withrington PG. A comparison of the effects of bradykinin, 5-hydroxytryptamine and histamine on the hepatic arterial and portal venous vascular beds of the dog: histamine H1 and H2-receptor populations. Br J Pharmacol. 1977; 60:123–33.
Article44. Cummings SA, Groszmann RJ, Kaumann AJ. Hypersensitivity of mesenteric veins to 5-hydroxytryptamine- and ketanserin-induced reduction of portal pressure in portal hypertensive rats. Br J Pharmacol. 1986; 89:501–13.
Article45. Cummings JL, Cilento EV, Reilly FD. Hepatic microvascular regulatory mechanisms. XII. Effects of 5-HT2-receptor blockade on serotonin-induced intralobular hypoperfusion. Int J Microcirc Clin Exp. 1993; 13:99–112.46. Kulinskii AS, Saratikov AS, Vstavskaia IuA, Udovitsina TI. Effect of substances altering the metabolism of endogenous serotonin on mitotic activity in the regenerating liver of mice. Farmakol Toksikol. 1983; 46:92–5.47. Kulinskii VI, Saratikov AS, Vstavskaia IuA, Udovitsina TI. Receptors mediating serotonin-stimulating liver regeneration in mice. Biull Eksp Biol Med. 1983; 95:89–91.48. Papadimas GK, Tzirogiannis KN, Mykoniatis MG, Grypioti AD, Manta GA, Panoutsopoulos GI. The emerging role of serotonin in liver regeneration. Swiss Med Wkly. 2012; 142:w13548.
Article49. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006; 312:104–7.
Article50. Ebrahimkhani MR, Oakley F, Murphy LB, Mann J, Moles A, Perugorria MJ, et al. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med. 2011; 17:1668–73.
Article51. Fang Y, Liu C, Shu B, Zhai M, Deng C, He C, et al. Axis of serotonin: pERK-YAP in liver regeneration. Life Sci. 2018; 209:490–7.
Article52. Starlinger P, Pereyra D, Hackl H, Ortmayr G, Braunwarth E, Santol J, et al. Consequences of perioperative serotonin reuptake inhibitor treatment during hepatic surgery. Hepatology. 2021; 73:1956–66.
Article53. Moore MC, DiCostanzo CA, Dardevet D, Lautz M, Farmer B, Neal DW, et al. Portal infusion of a selective serotonin reuptake inhibitor enhances hepatic glucose disposal in conscious dogs. Am J Physiol Endocrinol Metab. 2004; 287:E1057–63.
Article54. Haub S, Ritze Y, Ladel I, Saum K, Hubert A, Spruss A, et al. Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice. J Pharmacol Exp Ther. 2011; 339:790–8.
Article55. Choi W, Namkung J, Hwang I, Kim H, Lim A, Park HJ, et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2018; 9:4824.
Article56. Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006; 169:861–76.
Article57. Kim DC, Jun DW, Kwon YI, Lee KN, Lee HL, Lee OY, et al. 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis. Liver Int. 2013; 33:535–43.
Article58. Choi WG, Choi W, Oh TJ, Cha HN, Hwang I, Lee YK, et al. Inhibiting serotonin signaling through HTR2B in visceral adipose tissue improves obesity related insulin resistance. J Clin Invest. 2021. Oct. 7. [Epub]. https://doi.org/10.1172/JCI145331 .
Article59. Wegermann K, Howe C, Henao R, Wang Y, Guy CD, Abdelmalek MF, et al. Serum bile acid, vitamin E, and serotonin metabolites are associated with future liver-related events in nonalcoholic fatty liver disease. Hepatol Commun. 2021; 5:608–17.
Article60. Binetti J, Bertran L, Riesco D, Aguilar C, Martinez S, Sabench F, et al. Deregulated serotonin pathway in women with morbid obesity and NAFLD. Life (Basel). 2020; 10:245.
Article61. Pantano L, Agyapong G, Shen Y, Zhuo Z, Fernandez-Albert F, Rust W, et al. Molecular characterization and cell type composition deconvolution of fibrosis in NAFLD. Sci Rep. 2021; 11:18045.
Article62. Horst DA, Grace ND, LeCompte PM. Prolonged cholestasis and progressive hepatic fibrosis following imipramine therapy. Gastroenterology. 1980; 79:550–4.
Article63. Le Bricquir Y, Larrey D, Blanc P, Pageaux GP, Michel H. Tianeptine: an instance of drug-induced hepatotoxicity predicted by prospective experimental studies. J Hepatol. 1994; 21:771–3.64. Levine B, Jenkins AJ, Queen M, Jufer R, Smialek JE. Distribution of venlafaxine in three postmortem cases. J Anal Toxicol. 1996; 20:502–5.
Article65. Vuppalanchi R, Hayashi PH, Chalasani N, Fontana RJ, Bonkovsky H, Saxena R, et al. Duloxetine hepatotoxicity: a case-series from the drug-induced liver injury network. Aliment Pharmacol Ther. 2010; 32:1174–83.
Article66. Ayyash A, Holloway AC. Fluoxetine-induced hepatic lipid accumulation is linked to elevated serotonin production. Can J Physiol Pharmacol. 2021; 99:983–8.
Article67. Pan SJ, Tan YL, Yao SW, Xin Y, Yang X, Liu J, et al. Fluoxetine induces lipid metabolism abnormalities by acting on the liver in patients and mice with depression. Acta Pharmacol Sin. 2018; 39:1463–72.
Article68. Tuccinardi D, Farr OM, Upadhyay J, Oussaada SM, Mathew H, Paschou SA, et al. Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: a six-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes Metab. 2019; 21:1487–92.
Article69. Cerpa V, Gonzalez A, Richerson GB. Diphtheria toxin treatment of Pet-1-Cre floxed diphtheria toxin receptor mice disrupts thermoregulation without affecting respiratory chemoreception. Neuroscience. 2014; 279:65–76.
Article70. Wyler SC, Lord CC, Lee S, Elmquist JK, Liu C. Serotonergic control of metabolic homeostasis. Front Cell Neurosci. 2017; 11:277.
Article71. Choi W, Moon JH, Kim H. Serotonergic regulation of energy metabolism in peripheral tissues. J Endocrinol. 2020; 245:R1–10.
Article72. Wang L, Fan X, Han J, Cai M, Wang X, Wang Y, et al. Gut-derived serotonin contributes to the progression of non-alcoholic steatohepatitis via the liver HTR2A/PPARγ2 pathway. Front Pharmacol. 2020; 11:553.
Article