Korean Circ J.
2021 Dec;51(12):943-960. 10.4070/kcj.2021.0291.
Biomaterials-based Approaches for Cardiac Regeneration
- Affiliations
-
- 1Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD, USA
- 2Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA
- KMID: 2523160
- DOI: http://doi.org/10.4070/kcj.2021.0291
Abstract
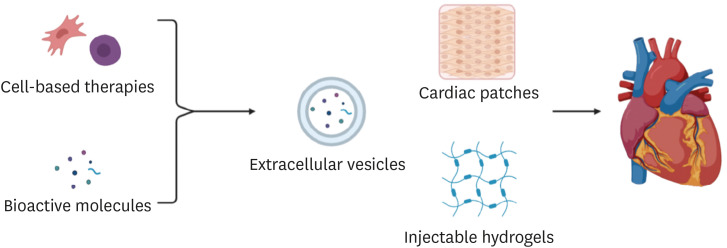
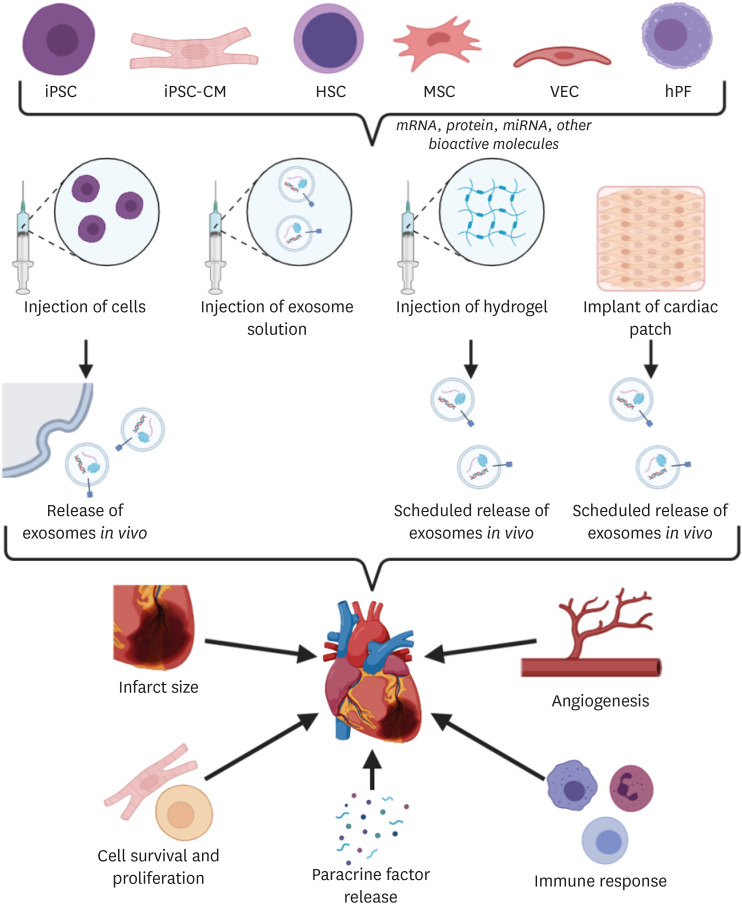
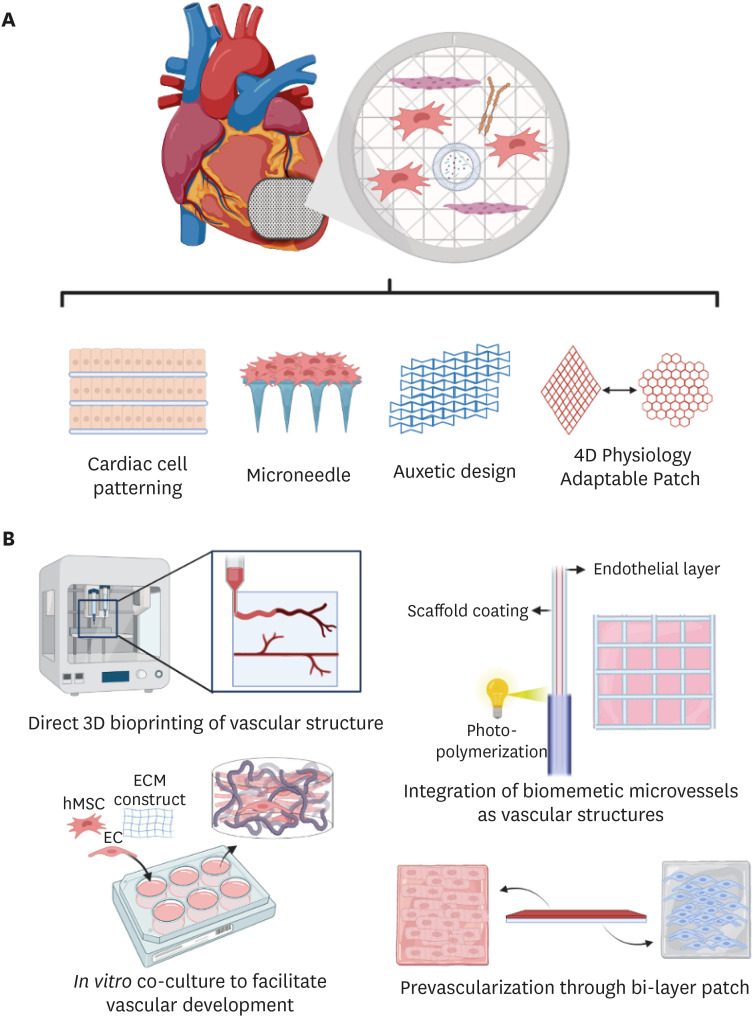
- The limited ability of cardiomyocytes to proliferate is a major cause of mortality and morbidity in cardiovascular diseases. There exist therapies for cardiac regeneration that are cell-based as well as that involve bioactive molecules. However, delivery remains one of the major challenges impeding such therapies from having clinical impact. Recent advancements in biomaterials-based approaches for cardiac regeneration have shown promise in clinical trials and animal studies in improving cardiac function, promoting angiogenesis, and reducing adverse immune response. This review will focus on current clinical studies of three contemporary biomaterials-based approaches for cardiac regeneration (extracellular vesicles, injectable hydrogels, and cardiac patches), remaining challenges and shortcomings to be overcome, and future directions for the use of biomaterials to promote cardiac regeneration.
Figure
Cited by 2 articles
-
Cardiovascular Regeneration via Stem Cells and Direct Reprogramming: A Review
Choon-Soo Lee, Joonoh Kim, Hyun-Jai Cho, Hyo-Soo Kim
Korean Circ J. 2022;52(5):341-353. doi: 10.4070/kcj.2022.0005.The Gut-Heart Axis: Updated Review for The Roles of Microbiome in Cardiovascular Health
Thi Van Anh Bui, Hyesoo Hwangbo, Yimin Lai, Seok Beom Hong, Yeon-Jik Choi, Hun-Jun Park, Kiwon Ban
Korean Circ J. 2023;53(8):499-518. doi: 10.4070/kcj.2023.0048.
Reference
-
1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021; 143:e254–e743. PMID: 33501848.2. He L, Zhou B. Cardiomyocyte proliferation: remove brakes and push accelerators. Cell Res. 2017; 27:959–960. PMID: 28707671.
Article3. Ludhwani D, Abraham J, Kanmanthareddy A. StatPearls. Heart transplantation rejection [Internet]. Treasure Island (FL): StatPearls Publishing;2021. 03. 12. cited 2021 July 22. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537057/.4. Bar A, Cohen S. Inducing endogenous cardiac regeneration: can biomaterials connect the dots? Front Bioeng Biotechnol. 2020; 8:126. PMID: 32175315.
Article5. Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007; 21:1345–1357. PMID: 17284483.
Article6. Uto K, Tsui JH, DeForest CA, Kim DH. Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Prog Polym Sci. 2017; 65:53–82. PMID: 28522885.
Article7. Williams MA, Mair DB, Lee W, Lee E, Kim DH. Engineering three-dimensional vascularized cardiac tissues. Tissue Eng Part B Rev. 2021; ten.teb.2020.0343.
Article8. Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012; 44:2060–2064. PMID: 22903023.
Article9. Kwon S, Shin S, Do M, et al. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J Control Release. 2021; 330:15–30. PMID: 33278480.
Article10. Yadid M, Lind JU, Ardoña HA, et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci Transl Med. 2020; 12:eaax8005. PMID: 33055246.
Article11. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200:373–383. PMID: 23420871.
Article12. Chun C, Smith AS, Kim H, et al. Astrocyte-derived extracellular vesicles enhance the survival and electrophysiological function of human cortical neurons in vitro. Biomaterials. 2021; 271:120700. PMID: 33631652.
Article13. Liu B, Lee BW, Nakanishi K, et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018; 2:293–303. PMID: 30271672.
Article14. Mackie AR, Klyachko E, Thorne T, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012; 111:312–321. PMID: 22581926.
Article15. Beltrami C, Besnier M, Shantikumar S, et al. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol Ther. 2017; 25:679–693. PMID: 28159509.
Article16. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (Amst). 2010; 4:214–222.
Article17. Antes TJ, Middleton RC, Luther KM, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology. 2018; 16:61. PMID: 30165851.
Article18. Wang X, Chen Y, Zhao Z, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018; 7:e008737. PMID: 30371236.
Article19. Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017; 38:201–211. PMID: 28158410.
Article20. Gao L, Wang L, Wei Y, et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med. 2020; 12:eaay1318. PMID: 32938792.
Article21. Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017; 44:2105–2116. PMID: 29241208.
Article22. Hirai K, Ousaka D, Fukushima Y, et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci Transl Med. 2020; 12:eabb3336. PMID: 33298561.
Article23. Hamada T, Dubois JL, Bellamy V, et al. In vitro controlled release of extracellular vesicles for cardiac repair from poly(glycerol sebacate) acrylate-based polymers. Acta Biomater. 2020; 115:92–103. PMID: 32814141.
Article24. Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005; 97:8–15. PMID: 16002755.
Article25. Huang K, Ozpinar EW, Su T, et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med. 2020; 12:9683.
Article26. Qian Z, Sharma D, Jia W, Radke D, Kamp T, Zhao F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics. 2019; 9:2143–2157. PMID: 31149034.
Article27. Shah M, Kc P, Zhang G. In vivo assessment of decellularized porcine myocardial slice as an acellular cardiac patch. ACS Appl Mater Interfaces. 2019; 11:23893–23900. PMID: 31188555.
Article28. Chen J, Zhan Y, Wang Y, et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018; 80:154–168. PMID: 30218777.
Article29. Pok S, Benavides OM, Hallal P, Jacot JG. Use of myocardial matrix in a chitosan-based full-thickness heart patch. Tissue Eng Part A. 2014; 20:1877–1887. PMID: 24433519.
Article30. Kapnisi M, Mansfield C, Marijon C, et al. Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction. Adv Funct Mater. 2018; 28:1800618. PMID: 29875619.
Article31. Hosoyama K, Ahumada M, McTiernan CD, et al. Nanoengineered electroconductive collagen-based cardiac patch for infarcted myocardium repair. ACS Appl Mater Interfaces. 2018; 10:44668–44677. PMID: 30508481.
Article32. Lakshmanan R, Kumaraswamy P, Krishnan UM, Sethuraman S. Engineering a growth factor embedded nanofiber matrix niche to promote vascularization for functional cardiac regeneration. Biomaterials. 2016; 97:176–195. PMID: 27177129.
Article33. O’Neill HS, O’Sullivan J, Porteous N, et al. A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J Tissue Eng Regen Med. 2018; 12:e384–e394. PMID: 27943590.
Article34. Serpooshan V, Zhao M, Metzler SA, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013; 34:9048–9055. PMID: 23992980.
Article35. Cui H, Liu C, Esworthy T, et al. 4D physiologically adaptable cardiac patch: a 4-month in vivo study for the treatment of myocardial infarction. Sci Adv. 2020; 6:eabb5067. PMID: 32637623.
Article36. Gao L, Kupfer ME, Jung JP, et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ Res. 2017; 120:1318–1325. PMID: 28069694.
Article37. Wang QL, Wang HJ, Li ZH, Wang YL, Wu XP, Tan YZ. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J Cell Mol Med. 2017; 21:1751–1766. PMID: 28244640.
Article38. Abbasgholizadeh R, Islas JF, Navran S, Potaman VN, Schwartz RJ, Birla RK. A highly conductive 3D cardiac patch fabricated using cardiac myocytes reprogrammed from human adipogenic mesenchymal stem cells. Cardiovasc Eng Technol. 2020; 11:205–218. PMID: 31916039.
Article39. Ye L, Chang YH, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014; 15:750–761. PMID: 25479750.
Article40. Mehrotra S, de Melo BA, Hirano M, et al. Nonmulberry silk based ink for fabricating mechanically robust cardiac patches and endothelialized myocardium-on-a-chip application. Adv Funct Mater. 2020; 30:1907436. PMID: 33071707.
Article41. Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005; 23:47–55. PMID: 15637621.
Article42. Chen WL, Kan CD. Using cell-seeded electrospun patch for myocardial injury: in-vitro and in rat model. Annu Int Conf IEEE Eng Med Biol Soc. 2018; 2018:5338–5341. PMID: 30441542.
Article43. Rai R, Tallawi M, Barbani N, et al. Biomimetic poly(glycerol sebacate) (PGS) membranes for cardiac patch application. Mater Sci Eng C. 2013; 33:3677–3687.
Article44. Ravichandran R, Venugopal JR, Mukherjee S, Sundarrajan S, Ramakrishna S. Elastomeric core/shell nanofibrous cardiac patch as a biomimetic support for infarcted porcine myocardium. Tissue Eng Part A. 2015; 21:1288–1298. PMID: 25559869.
Article45. Cristallini C, Vaccari G, Barbani N, et al. Cardioprotection of PLGA/gelatine cardiac patches functionalised with adenosine in a large animal model of ischaemia and reperfusion injury: a feasibility study. J Tissue Eng Regen Med. 2019; 13:1253–1264. PMID: 31050859.
Article46. Bahrami S, Solouk A, Mirzadeh H, Seifalian AM. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos, Part B Eng. 2019; 168:421–431.
Article47. Chung HJ, Kim JT, Kim HJ, et al. Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J Control Release. 2015; 205:218–230. PMID: 25681051.
Article48. Spadaccio C, Nappi F, De Marco F, et al. Implantation of a poly-L-lactide GCSF-functionalized scaffold in a model of chronic myocardial infarction. J Cardiovasc Transl Res. 2017; 10:47–65. PMID: 28116550.
Article49. Pushp P, Bhaskar R, Kelkar S, Sharma N, Pathak D, Gupta MK. Plasticized poly(vinylalcohol) and poly(vinylpyrrolidone) based patches with tunable mechanical properties for cardiac tissue engineering applications. Biotechnol Bioeng. 2021; 118:2312–2325. PMID: 33675237.
Article50. Su T, Huang K, Daniele MA, et al. Cardiac stem cell patch integrated with microengineered blood vessels promotes cardiomyocyte proliferation and neovascularization after acute myocardial infarction. ACS Appl Mater Interfaces. 2018; 10:33088–33096. PMID: 30188113.
Article51. Tang J, Wang J, Huang K, et al. Cardiac cell-integrated microneedle patch for treating myocardial infarction. Sci Adv. 2018; 4:eaat9365. PMID: 30498778.
Article52. Park BW, Jung SH, Das S, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020; 6:eaay6994. PMID: 32284967.
Article53. Wang Q, Yang H, Bai A, et al. Functional engineered human cardiac patches prepared from nature's platform improve heart function after acute myocardial infarction. Biomaterials. 2016; 105:52–65. PMID: 27509303.
Article54. Weinberger F, Breckwoldt K, Pecha S, et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. 2016; 8:363ra148.
Article55. Gao L, Gregorich ZR, Zhu W, et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018; 137:1712–1730. PMID: 29233823.
Article56. Schaefer JA, Guzman PA, Riemenschneider SB, Kamp TJ, Tranquillo RT. A cardiac patch from aligned microvessel and cardiomyocyte patches. J Tissue Eng Regen Med. 2018; 12:546–556. PMID: 28875579.
Article57. Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci (Weinh). 2019; 6:1900344. PMID: 31179230.
Article58. Kim DH, Lipke EA, Kim P, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010; 107:565–570. PMID: 20018748.
Article59. Kshitiz AJ, Afzal J, Kim SY, Kim DH. A nanotopography approach for studying the structure-function relationships of cells and tissues. Cell Adhes Migr. 2015; 9:300–307.
Article60. Mengsteab PY, Uto K, Smith AS, et al. Spatiotemporal control of cardiac anisotropy using dynamic nanotopographic cues. Biomaterials. 2016; 86:1–10. PMID: 26874887.
Article61. Carson D, Hnilova M, Yang X, et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl Mater Interfaces. 2016; 8:21923–21932. PMID: 26866596.
Article62. Tsui JH, Janebodin K, Ieronimakis N, et al. Harnessing sphingosine-1 phosphate signaling and nanotopographical cuesto regulate skeletal muscle maturation and vascularization. ACS Nano. 2017; 11:11954–11968. PMID: 29156133.63. Kim DH, Kshitiz , Smith RR, et al. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr Biol. 2012; 4:1019–1033.
Article64. Lin YD, Ko MC, Wu ST, et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater Sci. 2014; 2:567–580. PMID: 26827729.
Article65. Macadangdang J, Lee HJ, Carson D, et al. Capillary force lithography for cardiac tissue engineering. J Vis Exp. 2014; e50039.
Article66. Kim P, Yuan A, Nam KH, Jiao A, Kim DH. Fabrication of poly(ethylene glycol): gelatin methacrylate composite nanostructures with tunable stiffness and degradation for vascular tissue engineering. Biofabrication. 2014; 6:024112. PMID: 24717683.
Article67. Uto K, Aoyagi T, Kim DH, Ebara M. Free-standing nanopatterned poly(ε-caprolactone) thin films as a multifunctional scaffold. IEEE Trans NanoTechnol. 2018; 17:389–392.
Article68. Penland N, Choi E, Perla M, Park J, Kim DH. Facile fabrication of tissue-engineered constructs using nanopatterned cell sheets and magnetic levitation. Nanotechnology. 2017; 28:075103. PMID: 28028248.
Article69. Jiao A, Trosper NE, Yang HS, et al. Thermoresponsive nanofabricated substratum for the engineering of three-dimensional tissues with layer-by-layer architectural control. ACS Nano. 2014; 8:4430–4439. PMID: 24628277.
Article70. Williams NP, Rhodehamel M, Yan C, et al. Engineering anisotropic 3D tubular tissues with flexible thermoresponsive nanofabricated substrates. Biomaterials. 2020; 240:119856. PMID: 32105818.
Article71. Malki M, Fleischer S, Shapira A, Dvir T. Gold nanorod-based engineered cardiac patch for suture-free engraftment by near IR. Nano Lett. 2018; 18:4069–4073. PMID: 29406721.
Article72. Smith AS, Yoo H, Yi H, et al. Micro- and nano-patterned conductive graphene-PEG hybrid scaffolds for cardiac tissue engineering. Chem Commun (Camb). 2017; 53:7412–7415. PMID: 28634611.
Article73. Tsui JH, Ostrovsky-Snider NA, Yama DMP, et al. Conductive silk-polypyrrole composite scaffolds with bioinspired nanotopographic cues for cardiac tissue engineering. J Mater Chem B. 2018; 6:7185–7196. PMID: 31448124.
Article74. Choi JS, Smith AS, Williams NP, et al. Nanopatterned Nafion microelectrode arrays for in vitro cardiac electrophysiology. Adv Funct Mater. 2020; 30:1910660. PMID: 33244297.
Article75. Smith AS, Choi E, Gray K, et al. NanoMEA: a tool for high-throughput, electrophysiological phenotyping of patterned excitable cells. Nano Lett. 2020; 20:1561–1570. PMID: 31845810.76. Choi JS, Lee HJ, Rajaraman S, Kim DH. Recent advances in three-dimensional microelectrode array technologies for in vitro and in vivo cardiac and neuronal interfaces. Biosens Bioelectron. 2021; 171:112687. PMID: 33059168.
Article77. Peña B, Laughter M, Jett S, et al. Injectable hydrogels for cardiac tissue engineering. Macromol Biosci. 2018; 18:e1800079. PMID: 29733514.
Article78. Pati F, Jang J, Ha DH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014; 5:3935. PMID: 24887553.
Article79. Li Z, Guan J. Hydrogels for cardiac tissue engineering. Polymers (Basel). 2011; 3:740–761.
Article80. Shin YJ, Shafranek RT, Tsui JH, Walcott J, Nelson A, Kim DH. 3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta Biomater. 2021; 119:75–88. PMID: 33166713.
Article81. Mandrycky C, Wang Z, Kim K, Kim DH 3rd. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016; 34:422–434. PMID: 26724184.
Article82. Das S, Jang J. 3D bioprinting and decellularized ECM-based biomaterials for in vitro CV tissue engineering. J 3D Printing Med. 2018; 2:69–87.83. Anker SD, Coats AJ, Cristian G, et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J. 2015; 36:2297–2309. PMID: 26082085.
Article84. Lee LC, Wall ST, Klepach D, et al. Algisyl-LVR™ with coronary artery bypass grafting reduces left ventricular wall stress and improves function in the failing human heart. Int J Cardiol. 2013; 168:2022–2028. PMID: 23394895.
Article85. Rao SV, Zeymer U, Douglas PS, et al. Bioabsorbable intracoronary matrix for prevention of ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2016; 68:715–723. PMID: 27515331.
Article86. Traverse JH, Henry TD, Dib N, et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl Sci. 2019; 4:659–669. PMID: 31709316.
Article87. Tous E, Purcell B, Ifkovits JL, Burdick JA. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011; 4:528–542. PMID: 21710332.
Article88. Wang H, Zhou J, Liu Z, Wang C. Injectable cardiac tissue engineering for the treatment of myocardial infarction. J Cell Mol Med. 2010; 14:1044–1055. PMID: 20193036.
Article89. Contessotto P, Orbanić D, Da Costa M, et al. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci Transl Med. 2021; 13:eaaz5380. PMID: 33597263.
Article90. Chachques JC, Trainini JC, Lago N, Cortes-Morichetti M, Schussler O, Carpentier A. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg. 2008; 85:901–908. PMID: 18291168.
Article91. Hoeeg C, Dolatshahi-Pirouz A, Follin B. Injectable hydrogels for improving cardiac cell therapy-in vivo evidence and translational challenges. Gels. 2021; 7:7. PMID: 33499287.
Article92. Tsui JH, Leonard A, Camp ND, et al. Tunable electroconductive decellularized extracellular matrix hydrogels for engineering human cardiac microphysiological systems. Biomaterials. 2021; 272:120764. PMID: 33798964.
Article93. Liao X, Yang X, Deng H, et al. Injectable hydrogel-based nanocomposites for cardiovascular diseases. Front Bioeng Biotechnol. 2020; 8:251. PMID: 32296694.
Article94. Deng C, Zhang P, Vulesevic B, et al. A collagen–chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng Part A. 2010; 16:3099–3109. PMID: 20586613.
Article95. Song Y, Zhang C, Zhang J, et al. An injectable silk sericin hydrogel promotes cardiac functional recovery after ischemic myocardial infarction. Acta Biomater. 2016; 41:210–223. PMID: 27262742.
Article96. Wang H, Liu Z, Li D, et al. Injectable biodegradable hydrogels for embryonic stem cell transplantation: improved cardiac remodelling and function of myocardial infarction. J Cell Mol Med. 2012; 16:1310–1320. PMID: 21838774.
Article97. Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009; 54:1014–1023. PMID: 19729119.
Article98. Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res. 2016; 118:330–343. PMID: 26838317.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biomaterials and Futures for Bone Regeneration
- Engineering of Immune Microenvironment for Enhanced Tissue Remodeling
- Advancements and future directions in nerve and salivary gland treatments: a comprehensive review
- Bioengineering Approaches for Corneal Regenerative Medicine
- Current Status of Three-Dimensional Printing Inks for Soft Tissue Regeneration