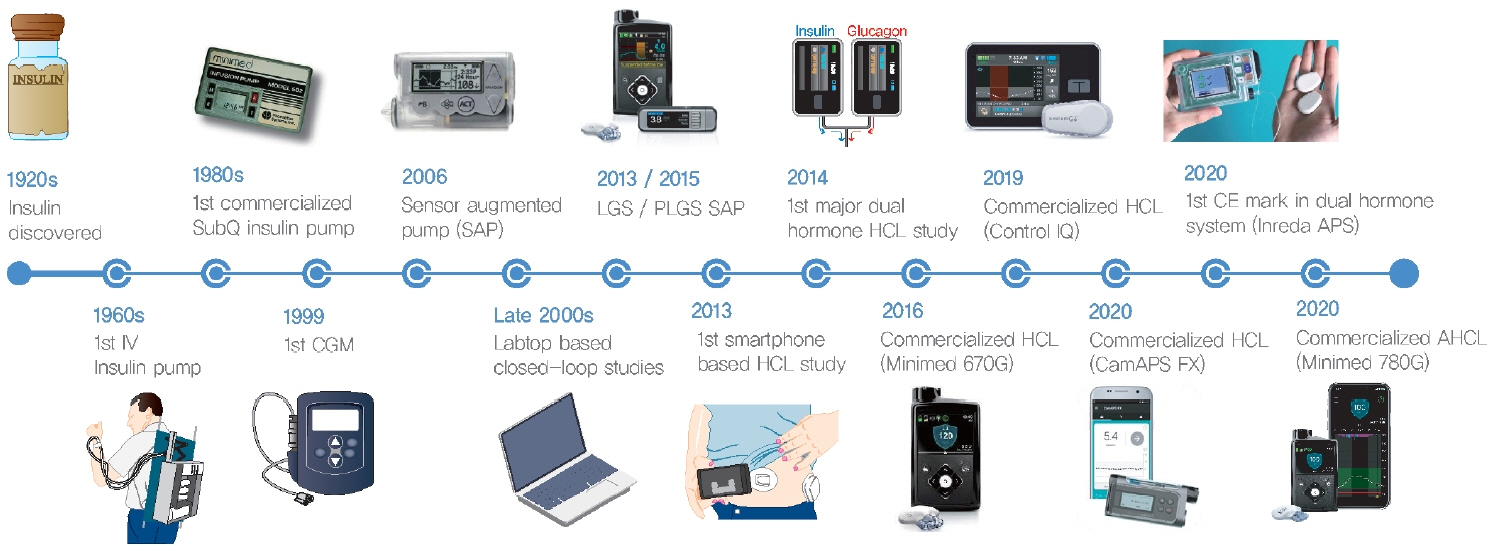
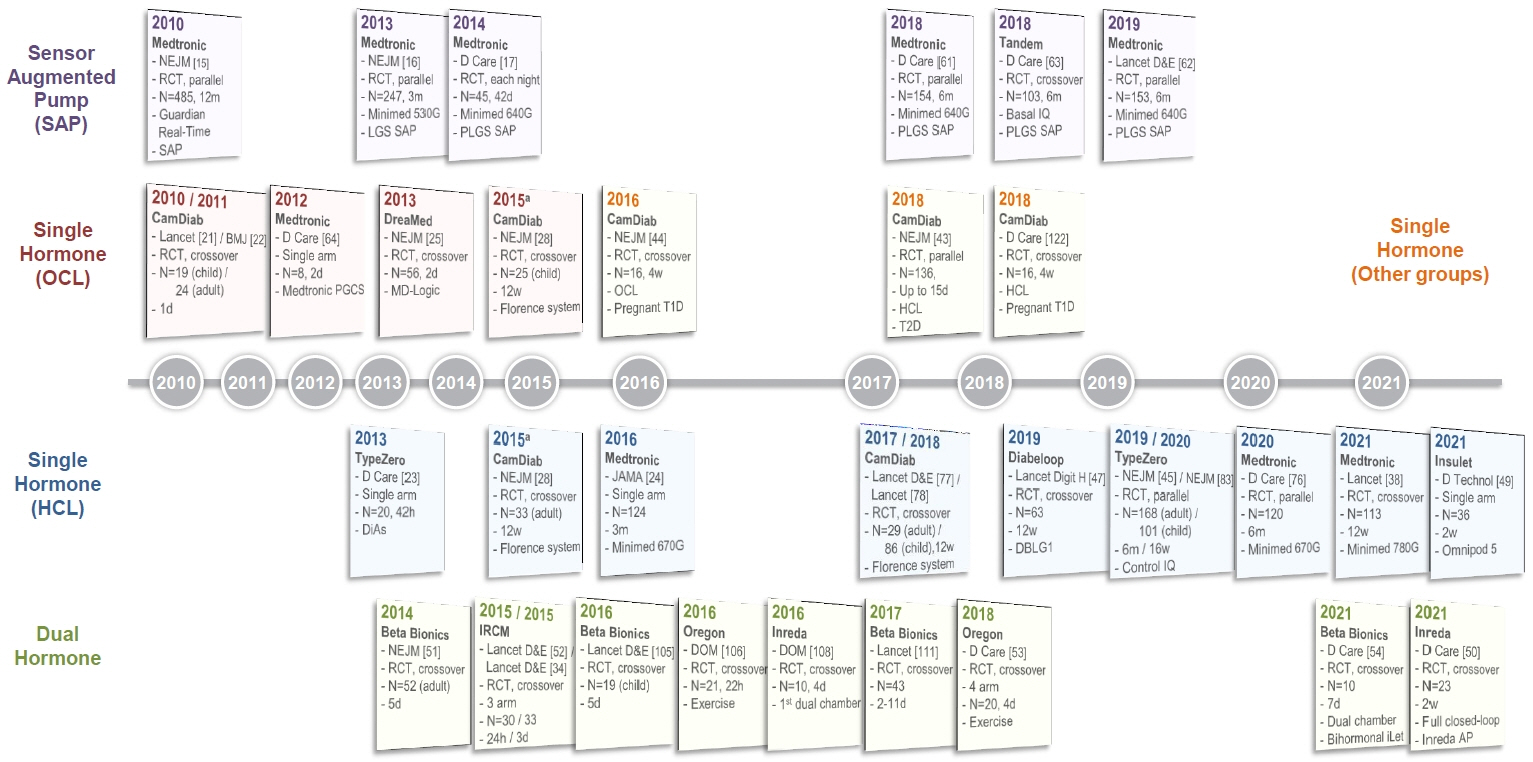
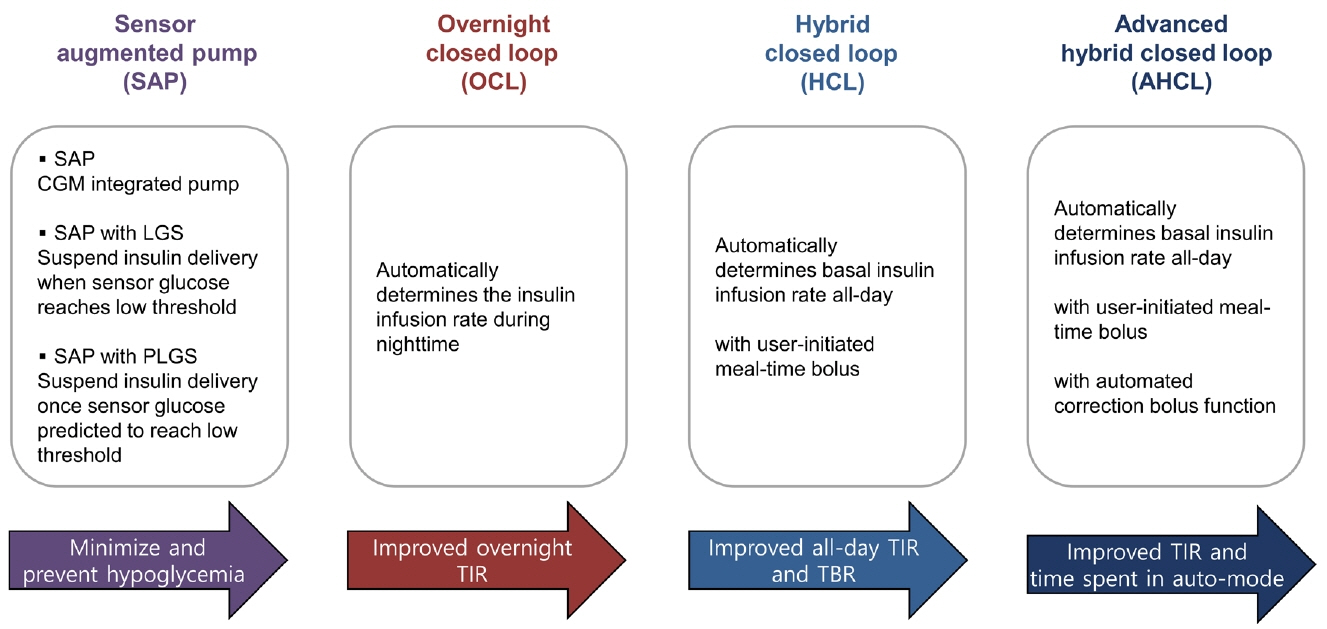
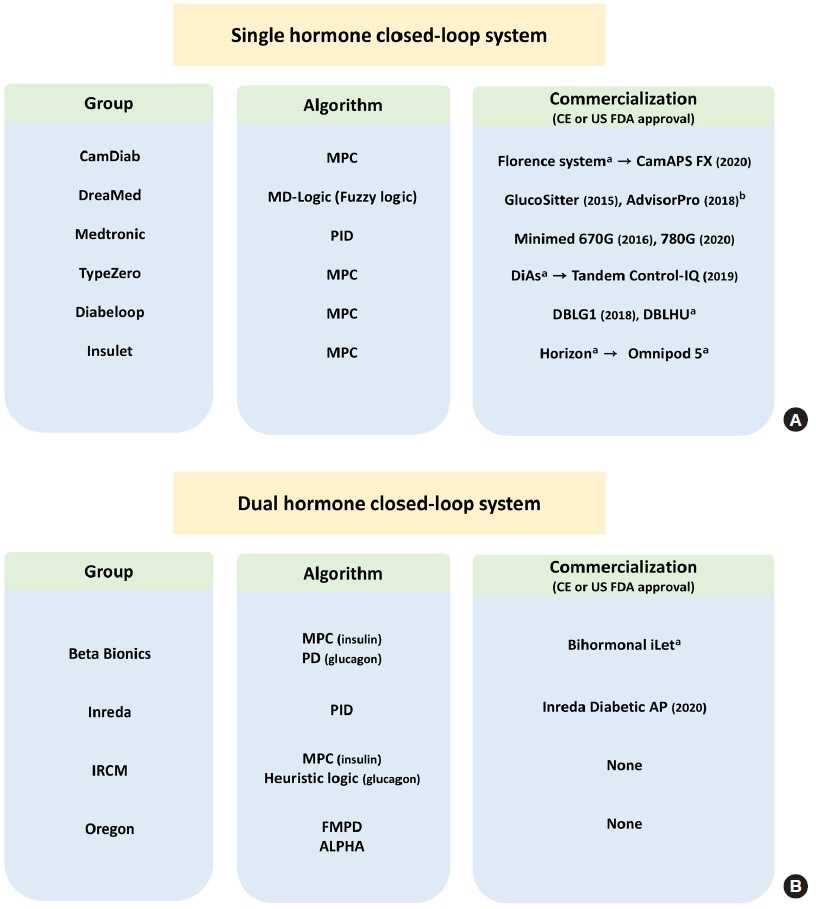
Diabetes Metab J.
2021 Nov;45(6):813-839. 10.4093/dmj.2021.0177.
Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2522722
- DOI: http://doi.org/10.4093/dmj.2021.0177
Abstract
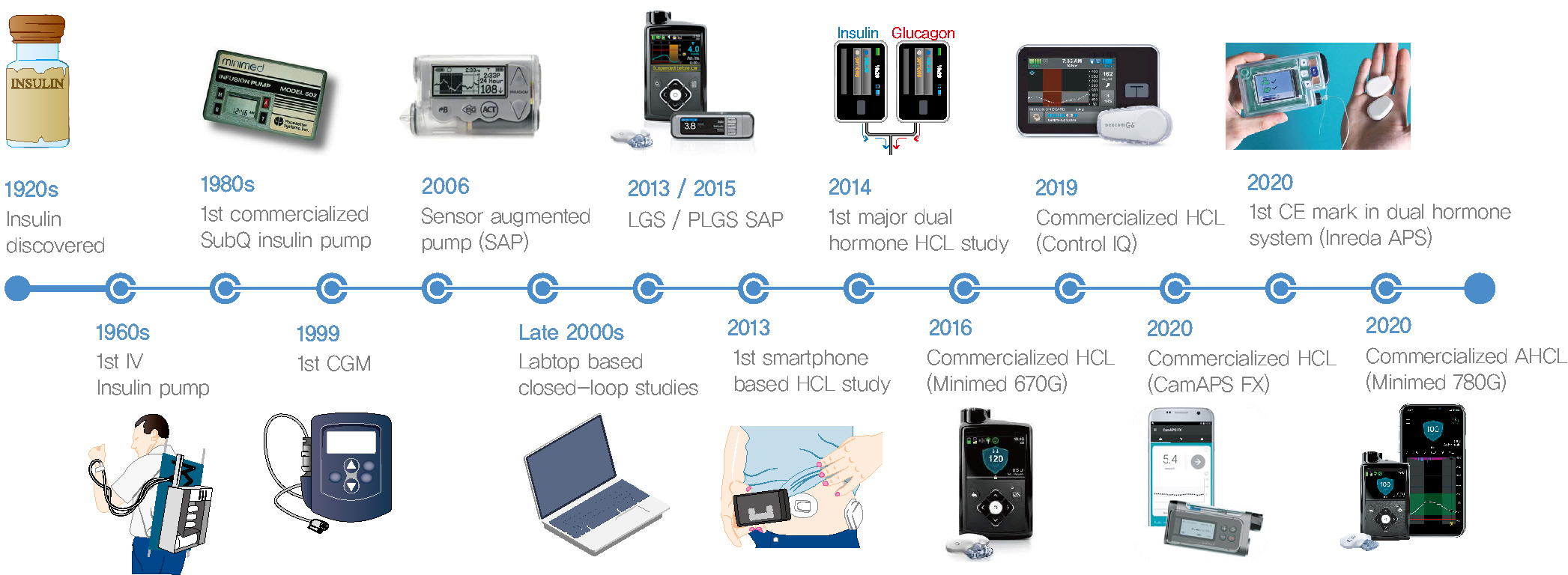
- Since Banting and Best isolated insulin in the 1920s, dramatic progress has been made in the treatment of type 1 diabetes mellitus (T1DM). However, dose titration and timely injection to maintain optimal glycemic control are often challenging for T1DM patients and their families because they require frequent blood glucose checks. In recent years, technological advances in insulin pumps and continuous glucose monitoring systems have created paradigm shifts in T1DM care that are being extended to develop artificial pancreas systems (APSs). Numerous studies that demonstrate the superiority of glycemic control offered by APSs over those offered by conventional treatment are still being published, and rapid commercialization and use in actual practice have already begun. Given this rapid development, keeping up with the latest knowledge in an organized way is confusing for both patients and medical staff. Herein, we explore the history, clinical evidence, and current state of APSs, focusing on various development groups and the commercialization status. We also discuss APS development in groups outside the usual T1DM patients and the administration of adjunct agents, such as amylin analogues, in APSs.
Keyword
Figure
Cited by 2 articles
-
Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes Metab J. 2023;47(1):27-41. doi: 10.4093/dmj.2022.0271.History of insulin treatment of pediatric patients with diabetes in Korea
Jae Hyun Kim, Choong Ho Shin, Sei Won Yang
Ann Pediatr Endocrinol Metab. 2021;26(4):237-241. doi: 10.6065/apem.2142242.121.
Reference
-
1. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018; 391:2449–62.
Article2. Chae HW, Seo GH, Song K, Choi HS, Suh J, Kwon A, et al. Incidence and prevalence of type 1 diabetes mellitus among Korean children and adolescents between 2007 and 2017: an epidemiologic study based on a national database. Diabetes Metab J. 2020; 44:866–74.
Article3. SEARCH for Diabetes in Youth Study Group, Liese AD, D’Agostino RB Jr, Hamman RF, Kilgo PD, Lawrence JM, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006; 118:1510–8.
Article4. Dayan CM, Korah M, Tatovic D, Bundy BN, Herold KC. Changing the landscape for type 1 diabetes: the first step to prevention. Lancet. 2019; 394:1286–96.
Article5. Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017; 357:j1321.
Article6. Cai X, Lin C, Yang W, Nie L, Ji L. Non-insulin antidiabetes treatment in type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab J. 2021; 45:312–25.
Article7. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019; 21:66–72.
Article8. Pettus JH, Zhou FL, Shepherd L, Preblick R, Hunt PR, Paranjape S, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care. 2019; 42:2220–7.
Article9. Hopkins D, Lawrence I, Mansell P, Thompson G, Amiel S, Campbell M, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care. 2012; 35:1638–42.
Article10. Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012; 366:1616–24.
Article11. Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017; 318:1358–66.
Article12. Blair JC, McKay A, Ridyard C, Thornborough K, Bedson E, Peak M, et al. Continuous subcutaneous insulin infusion versus multiple daily injection regimens in children and young people at diagnosis of type 1 diabetes: pragmatic randomized controlled trial and economic evaluation. BMJ. 2019; 365:l1226.13. Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012; 157:336–47.14. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J. 2019; 43:383–97.
Article15. Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulinpump therapy in type 1 diabetes. N Engl J Med. 2010; 363:311–20.
Article16. Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013; 369:224–32.
Article17. Maahs DM, Calhoun P, Buckingham BA, Chase HP, Hramiak I, Lum J, et al. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2014; 37:1885–91.
Article18. Buckingham BA, Raghinaru D, Cameron F, Bequette BW, Chase HP, Maahs DM, et al. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015; 38:1197–204.
Article19. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008; 31:934–9.
Article20. Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010; 33:1072–6.
Article21. Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010; 375:743–51.
Article22. Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011; 342:d1855.
Article23. Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care. 2013; 36:1851–8.
Article24. Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, et al. Safety of a hybrid closedloop insulin delivery system in patients with type 1 diabetes. JAMA. 2016; 316:1407–8.
Article25. Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013; 368:824–33.
Article26. Thabit H, Lubina-Solomon A, Stadler M, Leelarathna L, Walkinshaw E, Pernet A, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol. 2014; 2:701–9.
Article27. Kropff J, Del Favero S, Place J, Toffanin C, Visentin R, Monaro M, et al. 2 Month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol. 2015; 3:939–47.
Article28. Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015; 373:2129–40.
Article29. Elleri D, Allen JM, Kumareswaran K, Leelarathna L, Nodale M, Caldwell K, et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care. 2013; 36:838–44.
Article30. Ly TT, Roy A, Grosman B, Shin J, Campbell A, Monirabbasi S, et al. Day and night closed-loop control using the integrated medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015; 38:1205–11.
Article31. El-Khatib FH, Jiang J, Damiano ER. A feasibility study of bihormonal closed-loop blood glucose control using dual subcutaneous infusion of insulin and glucagon in ambulatory diabetic swine. J Diabetes Sci Technol. 2009; 3:789–803.
Article32. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010; 2:27ra27.
Article33. Castle JR, Engle JM, El Youssef J, Massoud RG, Yuen KC, Kagan R, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010; 33:1282–7.
Article34. Haidar A, Legault L, Matteau-Pelletier L, Messier V, Dallaire M, Ladouceur M, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015; 3:595–604.
Article35. Dovc K, Macedoni M, Bratina N, Lepej D, Nimri R, Atlas E, et al. Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: a randomised controlled crossover trial. Diabetologia. 2017; 60:2157–67.
Article36. Wilson LM, Jacobs PG, Ramsey KL, Resalat N, Reddy R, Branigan D, et al. Dual-hormone closed-loop system using a liquid stable glucagon formulation versus insulin-only closedloop system compared with a predictive low glucose suspend system: an open-label, outpatient, single-center, crossover, randomized controlled trial. Diabetes Care. 2020; 43:2721–9.37. Collyns OJ, Meier RA, Betts ZL, Chan DS, Frampton C, Frewen CM, et al. Improved glycemic outcomes with Medtronic MiniMed Advanced Hybrid Closed-Loop Delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021; 44:969–75.
Article38. Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021; 397:208–19.
Article39. Wilmot EG, Danne T. DIY artificial pancreas systems: the clinician perspective. Lancet Diabetes Endocrinol. 2020; 8:183–5.
Article40. Haidar A, Tsoukas MA, Bernier-Twardy S, Yale JF, Rutkowski J, Bossy A, et al. A novel dual-hormone insulin-and-pramlintide artificial pancreas for type 1 diabetes: a randomized controlled crossover trial. Diabetes Care. 2020; 43:597–606.
Article41. Ilkowitz JT, Katikaneni R, Cantwell M, Ramchandani N, Heptulla RA. Adjuvant liraglutide and insulin versus insulin monotherapy in the closed-loop system in type 1 diabetes: a randomized open-labeled crossover design trial. J Diabetes Sci Technol. 2016; 10:1108–14.42. Dassau E, Renard E, Place J, Farret A, Pelletier MJ, Lee J, et al. Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study. Diabetes Obes Metab. 2017; 19:1698–705.43. Bally L, Thabit H, Hartnell S, Andereggen E, Ruan Y, Wilinska ME, et al. Closed-loop insulin delivery for glycemic control in noncritical care. N Engl J Med. 2018; 379:547–56.
Article44. Stewart ZA, Wilinska ME, Hartnell S, Temple RC, Rayman G, Stanley KP, et al. Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med. 2016; 375:644–54.
Article45. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019; 381:1707–17.
Article46. Brown S, Raghinaru D, Emory E, Kovatchev B. First look at control-IQ: a new-generation automated insulin delivery system. Diabetes Care. 2018; 41:2634–36.
Article47. Benhamou PY, Franc S, Reznik Y, Thivolet C, Schaepelynck P, Renard E, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019; 1:e17–25.
Article48. Benhamou PY, Lablanche S, Vambergue A, Doron M, Franc S, Charpentier G. Patients with highly unstable type 1 diabetes eligible for islet transplantation can be managed with a closedloop insulin delivery system: a series of N-of-1 randomized controlled trials. Diabetes Obes Metab. 2021; 23:186–94.
Article49. Forlenza GP, Buckingham BA, Brown SA, Bode BW, Levy CJ, Criego AB, et al. First outpatient evaluation of a tubeless automated insulin delivery system with customizable glucose targets in children and adults with type 1 diabetes. Diabetes Technol Ther. 2021; 23:410–24.
Article50. Blauw H, Onvlee AJ, Klaassen M, van Bon AC, DeVries JH. Fully closed loop glucose control with a bihormonal artificial pancreas in adults with type 1 diabetes: an outpatient, randomized, crossover trial. Diabetes Care. 2021; 44:836–8.
Article51. Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014; 371:313–25.
Article52. Haidar A, Legault L, Messier V, Mitre TM, Leroux C, RabasaLhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015; 3:17–26.
Article53. Castle JR, El Youssef J, Wilson LM, Reddy R, Resalat N, Branigan D, et al. Randomized outpatient trial of single- and dualhormone closed-loop systems that adapt to exercise using wearable sensors. Diabetes Care. 2018; 41:1471–7.
Article54. Castellanos LE, Balliro CA, Sherwood JS, Jafri R, Hillard MA, Greaux E, et al. Performance of the insulin-only iLet bionic pancreas and the bihormonal iLet using dasiglucagon in adults with type 1 diabetes in a home-use setting. Diabetes Care. 2021; 44:e118–20.
Article55. Van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol. 2012; 6:1114–22.
Article56. Lal RA, Ekhlaspour L, Hood K, Buckingham B. Realizing a closed-loop (artificial pancreas) system for the treatment of type 1 diabetes. Endocr Rev. 2019; 40:1521–46.
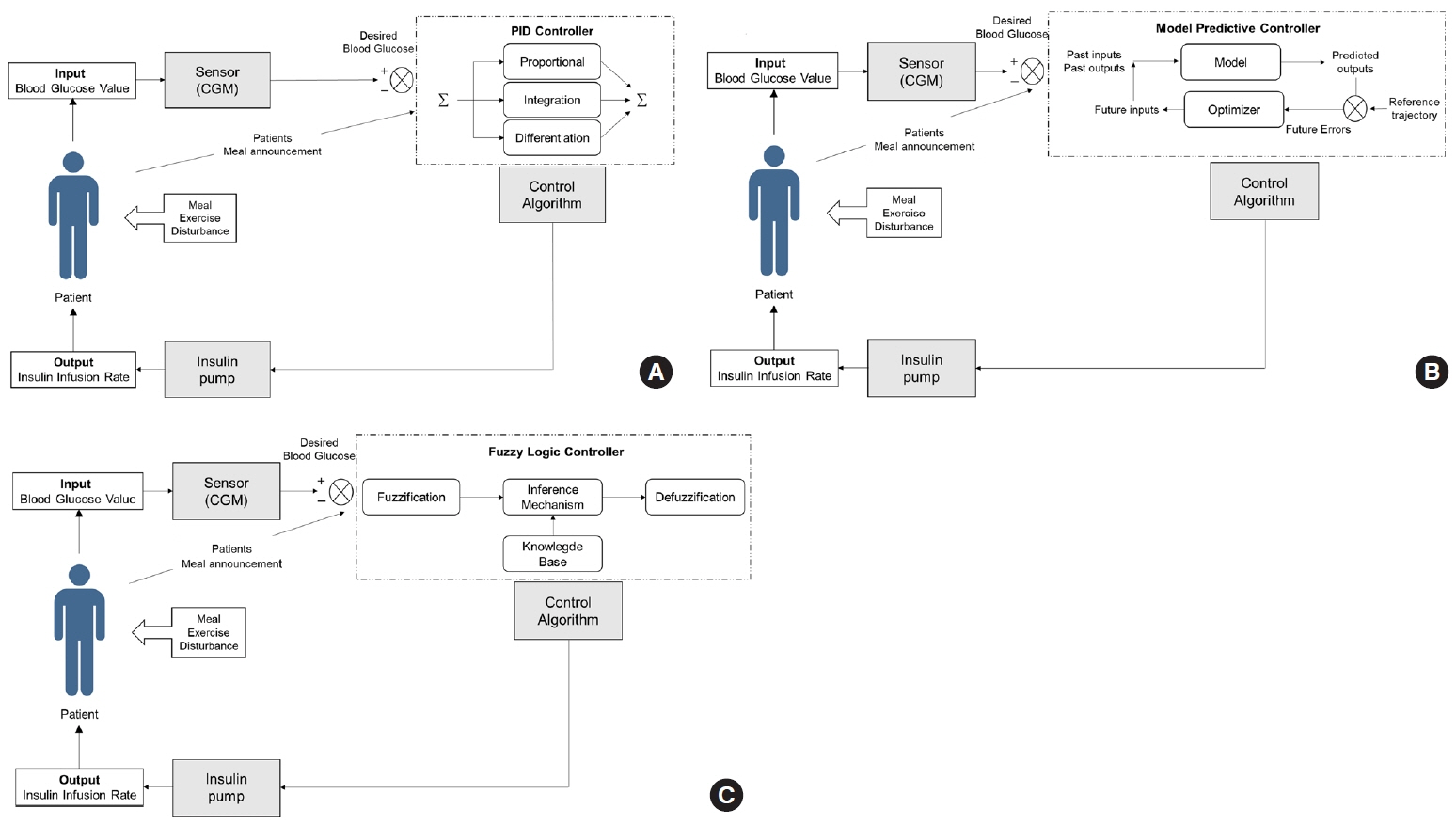
Article57. Unbehauen H. Control systems, robotics automation: system analysis control: classical approaches II. Oxford: Eolss Publisher;2009. Chapter 4, PID control. p. 58–79.58. Slover RH, Welsh JB, Criego A, Weinzimer SA, Willi SM, Wood MA, et al. Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. 2012; 13:6–11.
Article59. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013; 310:1240–7.60. Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care. 2017; 40:764–70.
Article61. Abraham MB, Nicholas JA, Smith GJ, Fairchild JM, King BR, Ambler GR, et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care. 2018; 41:303–10.
Article62. Bosi E, Choudhary P, de Valk HW, Lablanche S, Castaneda J, de Portu S, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 2019; 7:462–72.
Article63. Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018; 41:2155–61.
Article64. O’Grady MJ, Retterath AJ, Keenan DB, Kurtz N, Cantwell M, Spital G, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012; 35:2182–7.
Article65. Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-logic artificial pancreas in patients with type 1 diabetes: the DREAM Project. Diabetes Technol Ther. 2012; 14:728–35.
Article66. Ly TT, Breton MD, Keith-Hynes P, De Salvo D, Clinton P, Benassi K, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014; 37:2310–6.
Article67. Nimri R, Muller I, Atlas E, Miller S, Fogel A, Bratina N, et al. MD-logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014; 37:3025–32.
Article68. Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, ElKhairi R, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014; 37:1204–11.
Article69. Nimri R, Bratina N, Kordonouri O, Avbelj Stefanija M, Fath M, Biester T, et al. MD-Logic overnight type 1 diabetes control in home settings: a multicentre, multinational, single blind randomized trial. Diabetes Obes Metab. 2017; 19:553–61.
Article70. Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014; 37:1789–96.
Article71. Leelarathna L, Dellweg S, Mader JK, Allen JM, Benesch C, Doll W, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014; 37:1931–7.
Article72. Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, et al. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: a 3-week, free-living, randomized crossover trial. Diabetes Care. 2016; 39:2019–25.
Article73. Del Favero S, Boscari F, Messori M, Rabbone I, Bonfanti R, Sabbion A, et al. Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care. 2016; 39:1180–5.
Article74. Ly TT, Buckingham BA, DeSalvo DJ, Shanmugham S, SatinSmith M, DeBoer MD, et al. Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care. 2016; 39:e106–7.75. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, et al. Safety evaluation of the MiniMed 670G system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019; 21:11–9.
Article76. McAuley SA, Lee MH, Paldus B, Vogrin S, de Bock MI, Abraham MB, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020; 43:3024–33.
Article77. Bally L, Thabit H, Kojzar H, Mader JK, Qerimi-Hyseni J, Hartnell S, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017; 5:261–70.
Article78. Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018; 392:1321–9.
Article79. Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, et al. Home use of day-and-night hybrid closed-loop insulin delivery in very young children: a multicenter, 3-week, randomized trial. Diabetes Care. 2019; 42:594–600.
Article80. Anderson SM, Raghinaru D, Pinsker JE, Boscari F, Renard E, Buckingham BA, et al. Multinational home use of closed-loop control is safe and effective. Diabetes Care. 2016; 39:1143–50.
Article81. Kovatchev B, Cheng P, Anderson SM, Pinsker JE, Boscari F, Buckingham BA, et al. Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther. 2017; 19:18–24.
Article82. Brown SA, Beck RW, Raghinaru D, Buckingham BA, Laffel LM, Wadwa RP, et al. Glycemic outcomes of use of CLC versus PLGS in type 1 diabetes: a randomized controlled trial. Diabetes Care. 2020; 43:1822–8.
Article83. Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020; 383:836–45.
Article84. Biester T, Nir J, Remus K, Farfel A, Muller I, Biester S, et al. DREAM5: an open-label, randomized, cross-over study to evaluate the safety and efficacy of day and night closed-loop control by comparing the MD-Logic automated insulin delivery system to sensor augmented pump therapy in patients with type 1 diabetes at home. Diabetes Obes Metab. 2019; 21:822–8.
Article85. Quemerais MA, Doron M, Dutrech F, Melki V, Franc S, Antonakios M, et al. Preliminary evaluation of a new semiclosed-loop insulin therapy system over the prandial period in adult patients with type 1 diabetes: the WP6.0 Diabeloop study. J Diabetes Sci Technol. 2014; 8:1177–84.86. Benhamou PY, Huneker E, Franc S, Doron M, Charpentier G; Diabeloop Consortium. Customization of home closed-loop insulin delivery in adult patients with type 1 diabetes, assisted with structured remote monitoring: the pilot WP7 Diabeloop study. Acta Diabetol. 2018; 55:549–56.
Article87. Hanaire H, Franc S, Borot S, Penfornis A, Benhamou PY, Schaepelynck P, et al. Efficacy of the Diabeloop closed-loop system to improve glycaemic control in patients with type 1 diabetes exposed to gastronomic dinners or to sustained physical exercise. Diabetes Obes Metab. 2020; 22:324–34.
Article88. Amadou C, Franc S, Benhamou PY, Lablanche S, Huneker E, Charpentier G, et al. Diabeloop DBLG1 closed-loop system enables patients with type 1 diabetes to significantly improve their glycemic control in real-life situations without serious adverse events: 6-month follow-up. Diabetes Care. 2021; 44:844–6.
Article89. Del Favero S, Bruttomesso D, Di Palma F, Lanzola G, Visentin R, Filippi A, et al. First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care. 2014; 37:1212–5.
Article90. Buckingham BA, Christiansen MP, Forlenza GP, Wadwa RP, Peyser TA, Lee JB, et al. Performance of the Omnipod personalized model predictive control algorithm with meal bolus challenges in adults with type 1 diabetes. Diabetes Technol Ther. 2018; 20:585–95.
Article91. Forlenza GP, Buckingham BA, Christiansen MP, Wadwa RP, Peyser TA, Lee JB, et al. Performance of Omnipod personalized model predictive control algorithm with moderate intensity exercise in adults with type 1 diabetes. Diabetes Technol Ther. 2019; 21:265–72.
Article92. Sherr JL, Buckingham BA, Forlenza GP, Galderisi A, Ekhlaspour L, Wadwa RP, et al. Safety and performance of the Omnipod hybrid closed-loop system in adults, adolescents, and children with type 1 diabetes over 5 days under free-living conditions. Diabetes Technol Ther. 2020; 22:174–84.
Article93. Stone MP, Agrawal P, Chen X, Liu M, Shin J, Cordero TL, et al. Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther. 2018; 20:689–92.
Article94. Akturk HK, Giordano D, Champakanath A, Brackett S, Garg S, Snell-Bergeon J. Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab. 2020; 22:583–9.
Article95. Berget C, Messer LH, Vigers T, Frohnert BI, Pyle L, Wadwa RP, et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020; 21:310–8.
Article96. Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019; 42:2190–6.
Article97. Petrovski G, Al Khalaf F, Campbell J, Umer F, Almajaly D, Hamdan M, et al. One-year experience of hybrid closed-loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: drivers to successful outcomes. Acta Diabetol. 2021; 58:207–13.
Article98. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021; 23:601–8.
Article99. Messer LH, Berget C, Pyle L, Vigers T, Cobry E, Driscoll KA, et al. Real-world use of a new hybrid closed loop improves glycemic control in youth with type 1 diabetes. Diabetes Technol Ther. 2021. Jun. 21. [Epub]. https://doi.org/10.1089/dia.2021.0165.
Article100. Boughton CK, Hartnell S, Thabit H, Poettler T, Herzig D, Wilinska ME, et al. Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: a double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab. 2021; 23:1389–96.
Article101. Taborsky GJ Jr, Mundinger TO. Minireview: the role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology. 2012; 153:1055–62.
Article102. Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake: regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017; 13:133–48.103. Haidar A, Legault L, Dallaire M, Alkhateeb A, Coriati A, Messier V, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013; 185:297–305.
Article104. van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther. 2014; 16:131–6.
Article105. Russell SJ, Hillard MA, Balliro C, Magyar KL, Selagamsetty R, Sinha M, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016; 4:233–43.
Article106. Jacobs PG, El Youssef J, Reddy R, Resalat N, Branigan D, Condon J, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab. 2016; 18:1110–9.
Article107. Taleb N, Emami A, Suppere C, Messier V, Legault L, Ladouceur M, et al. Efficacy of single-hormone and dual-hormone artificial pancreas during continuous and interval exercise in adult patients with type 1 diabetes: randomised controlled crossover trial. Diabetologia. 2016; 59:2561–71.
Article108. Blauw H, van Bon AC, Koops R, DeVries JH; on behalf of the PCDIAB consortium. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016; 18:671–7.
Article109. Haidar A, Rabasa-Lhoret R, Legault L, Lovblom LE, Rakheja R, Messier V, et al. Single- and dual-hormone artificial pancreas for overnight glucose control in type 1 diabetes. J Clin Endocrinol Metab. 2016; 101:214–23.
Article110. Haidar A, Messier V, Legault L, Ladouceur M, Rabasa-Lhoret R. Outpatient 60-hour day-and-night glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or sensor-augmented pump therapy in adults with type 1 diabetes: an open-label, randomised, crossover, controlled trial. Diabetes Obes Metab. 2017; 19:713–20.
Article111. El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomized crossover trial. Lancet. 2017; 389:369–80.112. Taleb N, Haidar A, Messier V, Gingras V, Legault L, RabasaLhoret R. Glucagon in artificial pancreas systems: potential benefits and safety profile of future chronic use. Diabetes Obes Metab. 2017; 19:13–23.
Article113. Castle JR, El Youssef J, Bakhtiani PA, Cai Y, Stobbe JM, Branigan D, et al. Effect of repeated glucagon doses on hepatic glycogen in type 1 diabetes: implications for a bihormonal closed-loop system. Diabetes Care. 2015; 38:2115–9.
Article114. Kumareswaran K, Thabit H, Leelarathna L, Caldwell K, Elleri D, Allen JM, et al. Feasibility of closed-loop insulin delivery in type 2 diabetes: a randomized controlled study. Diabetes Care. 2014; 37:1198–203.
Article115. Thabit H, Hartnell S, Allen JM, Lake A, Wilinska ME, Ruan Y, et al. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 2017; 5:117–24.
Article116. Bally L, Gubler P, Thabit H, Hartnell S, Ruan Y, Wilinska ME, et al. Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Kidney Int. 2019; 96:593–6.117. Taleb N, Carpentier AC, Messier V, Ladouceur M, Haidar A, Rabasa-Lhoret R. Efficacy of artificial pancreas use in patients with type 2 diabetes using intensive insulin therapy: a randomized crossover pilot trial. Diabetes Care. 2019; 42:e107–9.
Article118. Marcinkevage JA, Narayan KM. Gestational diabetes mellitus: taking it to heart. Prim Care Diabetes. 2011; 5:81–8.
Article119. Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017; 390:2347–59.120. Murphy HR, Elleri D, Allen JM, Harris J, Simmons D, Rayman G, et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care. 2011; 34:406–11.
Article121. Murphy HR, Kumareswaran K, Elleri D, Allen JM, Caldwell K, Biagioni M, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care. 2011; 34:2527–9.
Article122. Stewart ZA, Wilinska ME, Hartnell S, O’Neil LK, Rayman G, Scott EM, et al. Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care. 2018; 41:1391–9.
Article123. Ahmed SH, Ewins DL, Bridges J, Timmis A, Payne N, Mooney C, et al. Do-it-yourself (DIY) artificial pancreas systems for type 1 diabetes: perspectives of two adult users, parent of a user and healthcare professionals. Adv Ther. 2020; 37:3929–41.124. Lewis D, Leibrand S; #OpenAPS Community. Real-world use of open source artificial pancreas systems. J Diabetes Sci Technol. 2016; 10:1411.
Article125. Lee JM, Newman MW, Gebremariam A, Choi P, Lewis D, Nordgren W, et al. Real-world use and self-reported health outcomes of a patient-designed do-it-yourself mobile technology system for diabetes: lessons for mobile health. Diabetes Technol Ther. 2017; 19:209–19.
Article126. Litchman ML, Lewis D, Kelly LA, Gee PM. Twitter analysis of #OpenAPS DIY artificial pancreas technology use suggests improved A1C and quality of life. J Diabetes Sci Technol. 2019; 13:164–70.
Article127. Hng TM, Burren D. Appearance of do-it-yourself closed-loop systems to manage type 1 diabetes. Intern Med J. 2018; 48:1400–4.
Article128. Holstein A, Bolli B. Normoglycaemic control with a selfmade fully closed-loop insulin delivery system during emergency surgery: an extemporaneous stress test. Acta Diabetol. 2019; 56:807–9.
Article129. Marshall DC, Holloway M, Korer M, Woodman J, Brackenridge A, Hussain S. Do-it-yourself artificial pancreas systems in type 1 diabetes: perspectives of two adult users, a caregiver and three physicians. Diabetes Ther. 2019; 10:1553–64.
Article130. Melmer A, Zuger T, Lewis DM, Leibrand S, Stettler C, Laimer M. Glycaemic control in individuals with type 1 diabetes using an open source artificial pancreas system (OpenAPS). Diabetes Obes Metab. 2019; 21:2333–7.
Article131. Braune K, O’Donnell S, Cleal B, Lewis D, Tappe A, Willaing I, et al. Real-world use of do-it-yourself artificial pancreas systems in children and adolescents with type 1 diabetes: online survey and analysis of self-reported clinical outcomes. JMIR Mhealth Uhealth. 2019; 7:e14087.
Article132. Braune K, May A, Thurm U. Safe and successful completion of a half marathon by an adult with type 1 diabetes using a personalized open source artificial pancreas system. J Diabetes Sci Technol. 2020; 14:1137–8.
Article133. Burnside M, Lewis D, Crocket H, Wilson R, Williman J, Jefferies C, et al. CREATE (Community deRivEd AutomaTEd insulin delivery) trial. Randomised parallel arm open label clinical trial comparing automated insulin delivery using a mobile controller (AnyDANA-loop) with an open-source algorithm with sensor augmented pump therapy in type 1 diabetes. J Diabetes Metab Disord. 2020; 19:1–15.
Article134. Lewis D. History and perspective on DIY closed looping. J Diabetes Sci Technol. 2019; 13:790–3.
Article135. Provenzano V, Guastamacchia E, Brancato D, Cappiello G, Maioli A, Mancini R, et al. Closing the loop with OpenAPS in people with type 1 diabetes: experience from Italy. Diabetes. 2018; 67(Suppl 1):993.
Article136. Choi SB, Hong ES, Noh YH. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes. 2018; 67(Suppl 1):964.
Article137. Jennings P, Hussain S. Do-it-yourself artificial pancreas systems: a review of the emerging evidence and insights for healthcare professionals. J Diabetes Sci Technol. 2020; 14:868–77.
Article138. Dowling L, Wilmot EG, Choudhary P. Do-it-yourself closedloop systems for people living with type 1 diabetes. Diabet Med. 2020; 37:1977–80.
Article139. Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R1475–84.
Article140. Schmitz O, Brock B, Rungby J. Amylin agonists: a novel approach in the treatment of diabetes. Diabetes. 2004; 53 Suppl 3:S233–8.
Article141. Hieronymus L, Griffin S. Role of amylin in type 1 and type 2 diabetes. Diabetes Educ. 2015; 41(1 Suppl):47S–56S.
Article142. Weinzimer SA, Sherr JL, Cengiz E, Kim G, Ruiz JL, Carria L, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012; 35:1994–9.
Article143. Sherr JL, Patel NS, Michaud CI, Palau-Collazo MM, Van Name MA, Tamborlane WV, et al. Mitigating meal-related glycemic excursions in an insulin-sparing manner during closed-loop insulin delivery: the beneficial effects of adjunctive pramlintide and liraglutide. Diabetes Care. 2016; 39:1127–34.
Article144. Riddle MC, Nahra R, Han J, Castle J, Hanavan K, Hompesch M, et al. Control of postprandial hyperglycemia in type 1 diabetes by 24-hour fixed-dose coadministration of pramlintide and regular human insulin: a randomized, two-way crossover study. Diabetes Care. 2018; 41:2346–52.
Article145. Taylor JR, Campbell KM. Diabetes drug update: how 4 new options stack up. J Fam Pract. 2007; 56:207–15.146. Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol. 2014; 76:535–59.
Article147. Boyle JG, Livingstone R, Petrie JR. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: a comparative review. Clin Sci (Lond). 2018; 132:1699–709.
Article148. Renukuntla VS, Ramchandani N, Trast J, Cantwell M, Heptulla RA. Role of glucagon-like peptide-1 analogue versus amylin as an adjuvant therapy in type 1 diabetes in a closed loop setting with ePID algorithm. J Diabetes Sci Technol. 2014; 8:1011–7.
Article149. Renard E. Insulin delivery route for the artificial pancreas: subcutaneous, intraperitoneal, or intravenous? Pros and cons. J Diabetes Sci Technol. 2008; 2:735–8.
Article150. van Dijk PR, Groenier KH, DeVries JH, Gans RO, Kleefstra N, Bilo HJ, et al. Continuous intraperitoneal insulin infusion versus subcutaneous insulin therapy in the treatment of type 1 diabetes: effects on glycemic variability. Diabetes Technol Ther. 2015; 17:379–84.
Article151. Logtenberg SJ, Kleefstra N, Houweling ST, Groenier KH, Gans RO, van Ballegooie E, et al. Improved glycemic control with intraperitoneal versus subcutaneous insulin in type 1 diabetes: a randomized controlled trial. Diabetes Care. 2009; 32:1372–7.
Article152. Liebl A, Hoogma R, Renard E, Geelhoed-Duijvestijn PH, Klein E, Diglas J, et al. A reduction in severe hypoglycaemia in type 1 diabetes in a randomized crossover study of continuous intraperitoneal compared with subcutaneous insulin infusion. Diabetes Obes Metab. 2009; 11:1001–8.
Article153. Jeandidier N, Selam JL, Renard E, Guerci B, Lassman-Vague V, Rocher L, et al. Decreased severe hypoglycemia frequency during intraperitoneal insulin infusion using programmable implantable pumps. Evadiac Study Group. Diabetes Care. 1996; 19:780.154. Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care. 2010; 33:121–7.
Article155. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006; 55:44–50.
Article156. Dassau E, Zisser H, Harvey RA, Percival MW, Grosman B, Bevier W, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013; 36:801–9.
Article157. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013; 15:386–400.
Article158. Horowitz ME, Kaye WA, Pepper GM, Reynolds KE, Patel SR, Knudson KC, et al. An analysis of Medtronic MiniMed 670G insulin pump use in clinical practice and the impact on glycemic control, quality of life, and compliance. Diabetes Res Clin Pract. 2021; 177:108876.
Article159. Grosman B, Wu D, Parikh N, Roy A, Voskanyan G, Kurtz N, et al. Fast-acting insulin aspart (Fiasp(R)) improves glycemic outcomes when used with MiniMedTM 670G hybrid closedloop system in simulated trials compared to NovoLog(R). Comput Methods Programs Biomed. 2021; 205:106087.160. Alfonsi JE, Choi EE, Arshad T, Sammott SS, Pais V, Nguyen C, et al. Carbohydrate counting App using image recognition for youth with type 1 diabetes: pilot randomized control trial. JMIR Mhealth Uhealth. 2020; 8:e22074.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Technology for Type 1 Diabetes
- Artificial Pancreas: A Concise Review
- In the Era of ChatGPT, Can Medical Artificial Intelligence Replace the Doctor?
- The imitation game: a review of the use of artificial intelligence in colonoscopy, and endoscopists’ perceptions thereof
- Role of artificial intelligence in diagnosing Barrett’s esophagus-related neoplasia