Endocrinol Metab.
2021 Oct;36(5):952-964. 10.3803/EnM.2021.1198.
Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions
- Affiliations
-
- 1Institute of Clinical Physiology, National Research Council of Italy (CNR), Pisa, Italy
- 2Fondazione CNR-Regione Toscana Gabriele Monasterio, Pisa, Italy
- KMID: 2521943
- DOI: http://doi.org/10.3803/EnM.2021.1198
Abstract
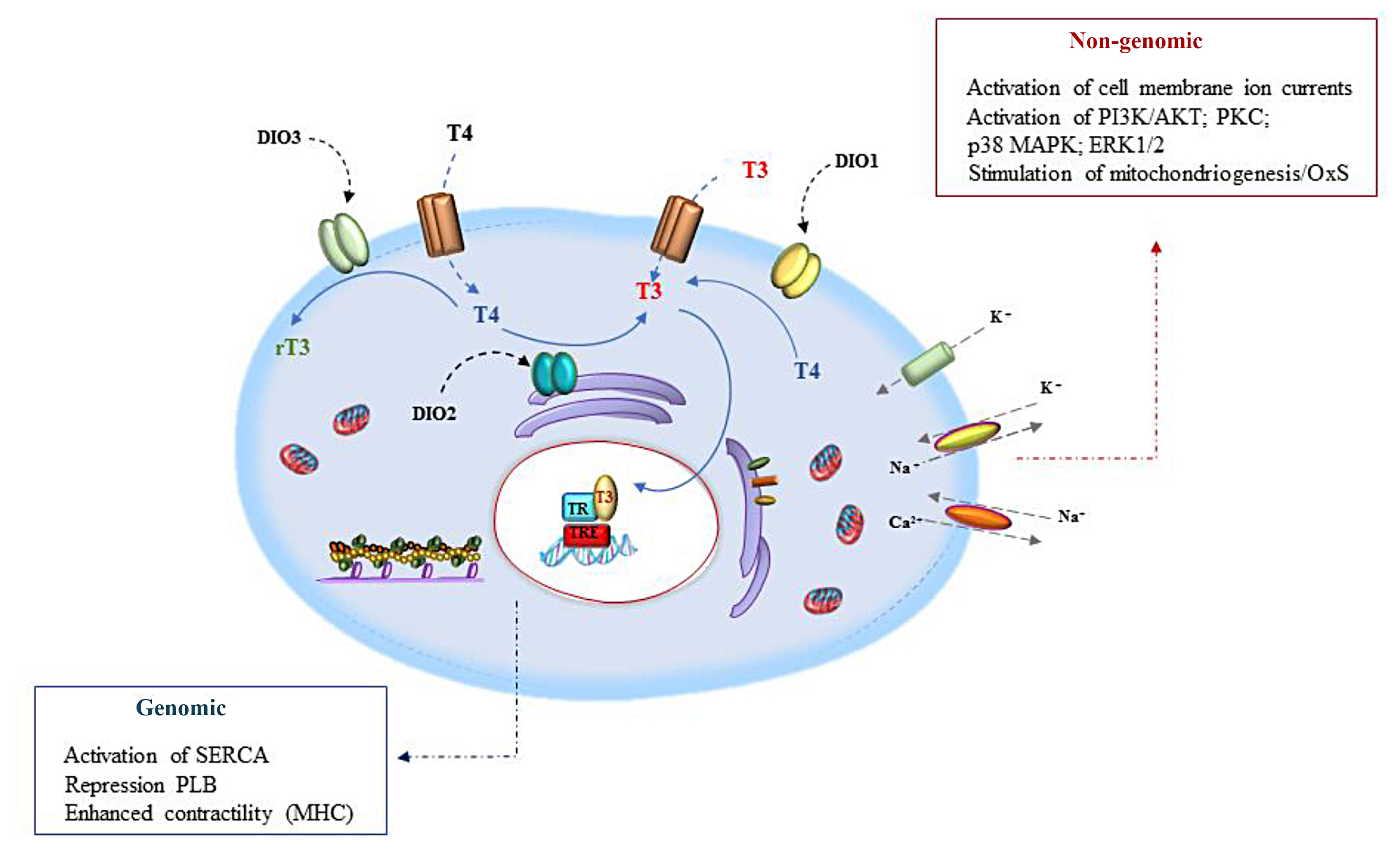
- Thyroid hormone (TH) signaling is strictly regulated by iodothyronine deiodinase activity, which both preserves the circulating levels of the biologically active triiodothyronine (T3) and regulates TH homeostasis at the local level, in a cell- and time-dependent manner. Three deiodinases have been identified—namely iodothyronine deiodinase 1 (DIO1), DIO2, and DIO3—that differ in their catalytic properties and tissue distribution. The deiodinases represent a dynamic system that changes in the different stages of life according to their functions and roles in various cell types and tissues. Deiodinase activity at the tissue level permits cell-targeted fine regulation of TH homeostasis, mediating the activation (DIO1 and DIO2) and inactivation (DIO3) of THs. Deiodinase homeostasis is the driving force that leads T3-target cells towards customized TH signaling, which takes into account both the hormonal circulating levels and the tissue-specific response. This review analyzes the complex role of deiodinases in physiological and pathological contexts, exploring new challenges and opportunities deriving from a deeper knowledge of the dynamics underlying their roles and functions.
Figure
Reference
-
1. Orozco A, Valverde-R C, Olvera A, Garcia-G C. Iodothyronine deiodinases: a functional and evolutionary perspective. J Endocrinol. 2012; 215:207–19.
Article2. Toyoda N, Berry MJ, Harney JW, Larsen PR. Topological analysis of the integral membrane protein, type 1 iodothyronine deiodinase (D1). J Biol Chem. 1995; 270:12310–8.
Article3. Zhang CY, Kim S, Harney JW, Larsen PR. Further characterization of thyroid hormone response elements in the human type 1 iodothyronine deiodinase gene. Endocrinology. 1998; 139:1156–63.
Article4. Gereben B, Salvatore D, Harney JW, Tu HM, Larsen PR. The human, but not rat, dio2 gene is stimulated by thyroid transcription factor-1 (TTF-1). Mol Endocrinol. 2001; 15:112–24.
Article5. Hernandez A, Fiering S, Martinez E, Galton VA, St Germain D. The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology. 2002; 143:4483–6.
Article6. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008; 29:898–938.
Article7. Callebaut I, Curcio-Morelli C, Mornon JP, Gereben B, Buettner C, Huang S, et al. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J Biol Chem. 2003; 278:36887–96.
Article8. Curcio-Morelli C, Gereben B, Zavacki AM, Kim BW, Huang S, Harney JW, et al. In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology. 2003; 144:937–46.
Article9. Sagar GD, Gereben B, Callebaut I, Mornon JP, Zeold A, Curcio-Morelli C, et al. The thyroid hormone-inactivating deiodinase functions as a homodimer. Mol Endocrinol. 2008; 22:1382–93.
Article10. Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000; 141:4309–12.
Article11. Baqui M, Botero D, Gereben B, Curcio C, Harney JW, Salvatore D, et al. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem. 2003; 278:1206–11.
Article12. Bianco AC, da Conceicao RR. The deiodinase trio and thyroid hormone signaling. Methods Mol Biol. 2018; 1801:67–83.
Article13. Larsen PR. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med. 1982; 306:23–32.14. Christoffolete MA, Ribeiro R, Singru P, Fekete C, da Silva WS, Gordon DF, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006; 147:1735–43.
Article15. Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci U S A. 2000; 97:1287–92.
Article16. de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001; 108:1379–85.
Article17. Bassett JH, Boyde A, Howell PG, Bassett RH, Galliford TM, Archanco M, et al. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc Natl Acad Sci U S A. 2010; 107:7604–9.
Article18. Marsili A, Tang D, Harney JW, Singh P, Zavacki AM, Dentice M, et al. Type II iodothyronine deiodinase provides intracellular 3,5,3′-triiodothyronine to normal and regenerating mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2011; 301:E818–24.
Article19. Bianco AC, Silva JE. Cold exposure rapidly induces virtual saturation of brown adipose tissue nuclear T3 receptors. Am J Physiol. 1988; 255(4 Pt 1):E496–503.
Article20. Freitas BC, Gereben B, Castillo M, Kallo I, Zeold A, Egri P, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest. 2010; 120:2206–17.
Article21. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002; 23:38–89.
Article22. Steinsapir J, Harney J, Larsen PR. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J Clin Invest. 1998; 102:1895–9.
Article23. Steinsapir J, Bianco AC, Buettner C, Harney J, Larsen PR. Substrate-induced down-regulation of human type 2 deiodinase (hD2) is mediated through proteasomal degradation and requires interaction with the enzyme’s active center. Endocrinology. 2000; 141:1127–35.
Article24. Kim SW, Harney JW, Larsen PR. Studies of the hormonal regulation of type 2 5′-iodothyronine deiodinase messenger ribonucleic acid in pituitary tumor cells using semiquantitative reverse transcription-polymerase chain reaction. Endocrinology. 1998; 139:4895–905.25. Sagar GD, Gereben B, Callebaut I, Mornon JP, Zeold A, da Silva WS, et al. Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol. 2007; 27:4774–83.
Article26. Gereben B, Goncalves C, Harney JW, Larsen PR, Bianco AC. Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol Endocrinol. 2000; 14:1697–708.
Article27. Kaplan MM, Yaskoski KA. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980; 66:551–62.
Article28. Huang TS, Chopra IJ, Beredo A, Solomon DH, Chua Teco GN. Skin is an active site for the inner ring monodeiodination of thyroxine to 3,3′,5′-triiodothyronine. Endocrinology. 1985; 117:2106–13.
Article29. Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003; 88:1384–8.
Article30. Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol. 2011; 209:273–82.31. Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007; 148:3080–8.
Article32. Fonseca TL, Werneck-De-Castro JP, Castillo M, Bocco BM, Fernandes GW, McAninch EA, et al. Tissue-specific inactivation of type 2 deiodinase reveals multilevel control of fatty acid oxidation by thyroid hormone in the mouse. Diabetes. 2014; 63:1594–604.
Article33. Fonseca TL, Fernandes GW, McAninch EA, Bocco BM, Abdalla SM, Ribeiro MO, et al. Perinatal deiodinase 2 expression in hepatocytes defines epigenetic susceptibility to liver steatosis and obesity. Proc Natl Acad Sci U S A. 2015; 112:14018–23.
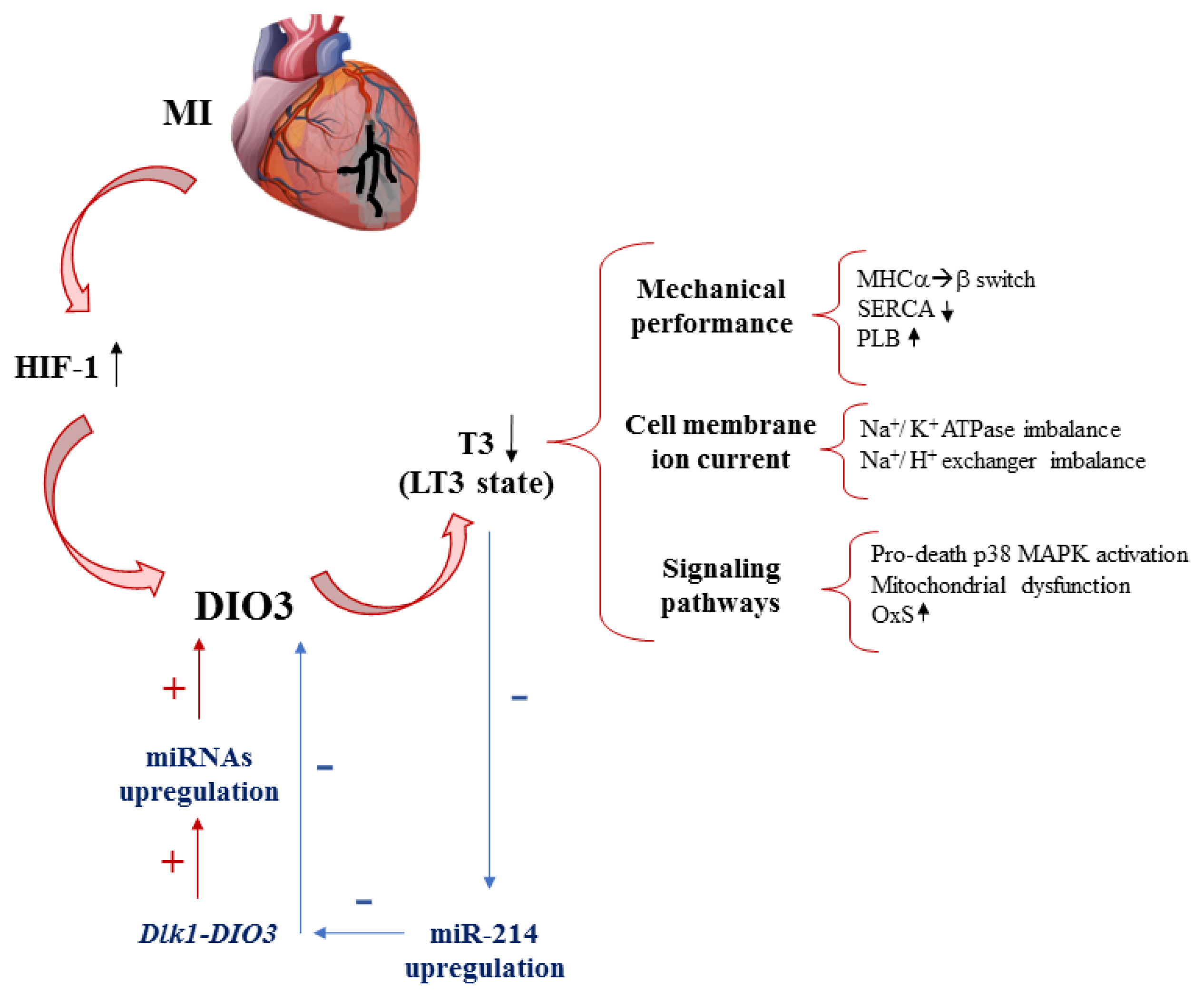
Article34. Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest. 2008; 118:975–83.
Article35. Lazar MA. Thyroid hormone action: a binding contract. J Clin Invest. 2003; 112:497–9.
Article36. Ojamaa K, Kenessey A, Klein I. Thyroid hormone regulation of phospholamban phosphorylation in the rat heart. Endocrinology. 2000; 141:2139–44.
Article37. Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol. 2008; 29:211–8.
Article38. Weitzel JM, Iwen KA, Seitz HJ. Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol. 2003; 88:121–8.
Article39. Iervasi G, Pingitore A, Gerdes AM, Razvi S. Thyroid and heart: a comprehensive translational essay. 2nd ed. Cham: Springer;2020. Chapter 21:TH treatment in patients with cardiac disorders: general aspects and rationale. p. 373–80.40. Wassen FW, Schiel AE, Kuiper GG, Kaptein E, Bakker O, Visser TJ, et al. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology. 2002; 143:2812–5.
Article41. Sabatino L, Iervasi G, Ferrazzi P, Francesconi D, Chopra IJ. A study of iodothyronine 5′-monodeiodinase activities in normal and pathological tissues in man and their comparison with activities in rat tissues. Life Sci. 2000; 68:191–202.
Article42. Trivieri MG, Oudit GY, Sah R, Kerfant BG, Sun H, Gramolini AO, et al. Cardiac-specific elevations in thyroid hormone enhance contractility and prevent pressure overload-induced cardiac dysfunction. Proc Natl Acad Sci U S A. 2006; 103:6043–8.
Article43. Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007; 12:331–43.
Article44. Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, et al. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res. 2001; 89:591–8.
Article45. Sabatino L, Kusmic C, Iervasi G. Modification of cardiac thyroid hormone deiodinases expression in an ischemia/reperfusion rat model after T3 infusion. Mol Cell Biochem. 2020; 475:205–14.
Article46. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136:215–33.
Article47. da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008; 24:306–16.
Article48. Janssen R, Zuidwijk M, Muller A, Mulders J, Oudejans CB, Simonides WS. Cardiac expression of deiodinase type 3 (Dio3) following myocardial infarction is associated with the induction of a pluripotency microRNA signature from the Dlk1-Dio3 genomic region. Endocrinology. 2013; 154:1973–8.
Article49. Janssen R, Zuidwijk MJ, Muller A, van Mil A, Dirkx E, Oudejans CB, et al. MicroRNA 214 is a potential regulator of thyroid hormone levels in the mouse heart following myocardial infarction, by targeting the thyroid-hormone-inactivating enzyme deiodinase type III. Front Endocrinol (Lausanne). 2016; 7:22.
Article50. Ronnebaum SM, Patterson C. The FoxO family in cardiac function and dysfunction. Annu Rev Physiol. 2010; 72:81–94.
Article51. Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013; 14:83–97.
Article52. Ferdous A, Wang ZV, Luo Y, Li DL, Luo X, Schiattarella GG, et al. FoxO1-Dio2 signaling axis governs cardiomyocyte thyroid hormone metabolism and hypertrophic growth. Nat Commun. 2020; 11:2551.
Article53. Fekete C, Gereben B, Doleschall M, Harney JW, Dora JM, Bianco AC, et al. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: implications for the nonthyroidal illness syndrome. Endocrinology. 2004; 145:1649–55.
Article54. Lamirand A, Pallud-Mothre S, Ramauge M, Pierre M, Courtin F. Oxidative stress regulates type 3 deiodinase and type 2 deiodinase in cultured rat astrocytes. Endocrinology. 2008; 149:3713–21.
Article55. Bianco AC, Dumitrescu A, Gereben B, Ribeiro MO, Fonseca TL, Fernandes GW, et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev. 2019; 40:1000–47.
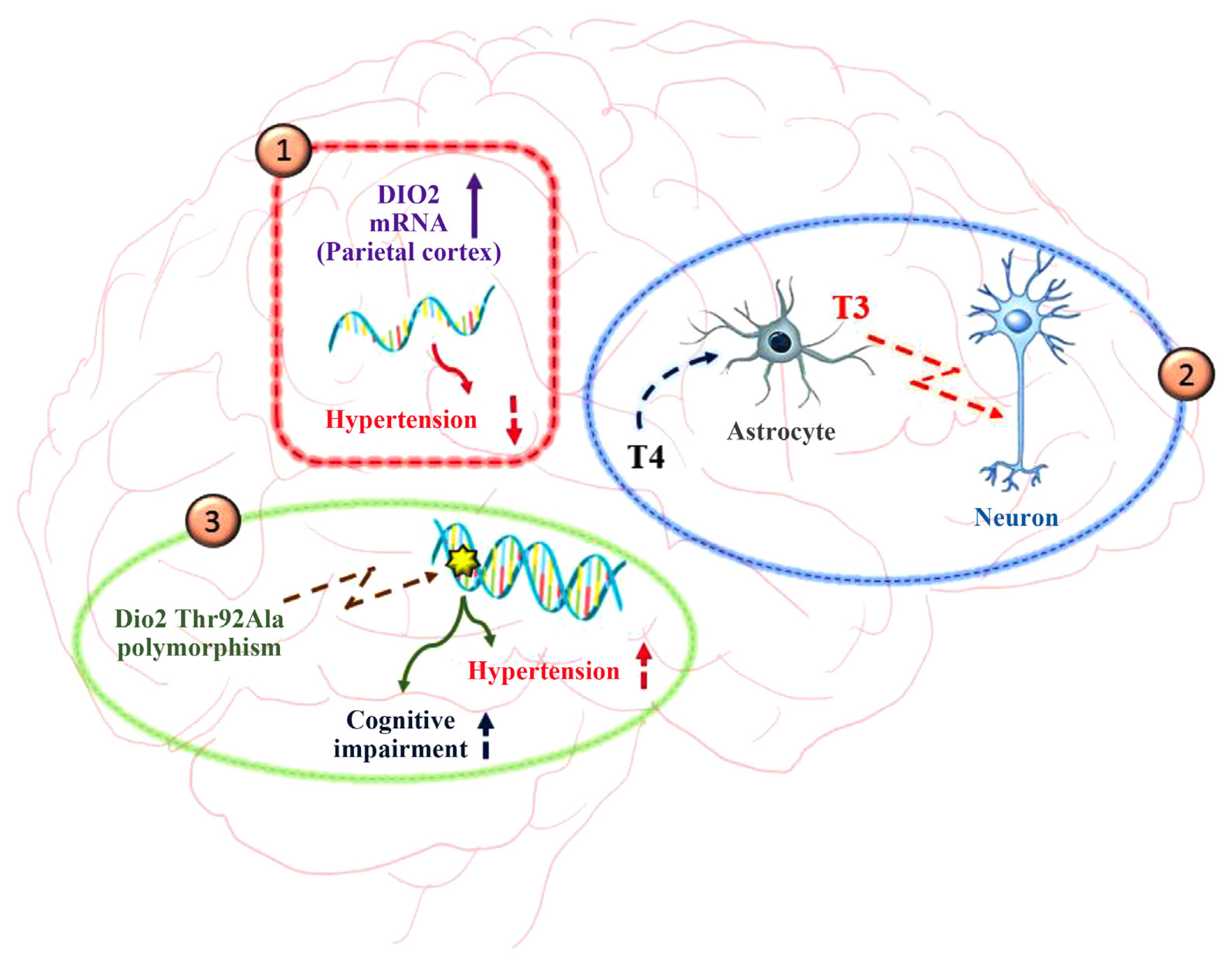
Article56. Sabatino L, Federighi G, Del Seppia C, Lapi D, Costagli C, Scuri R, et al. Thyroid hormone deiodinases response in brain of spontaneausly hypertensive rats after hypotensive effects induced by mandibular extension. Endocrine. 2021; 74:100–7.
Article57. Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, et al. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003; 88:2880–8.
Article58. McAninch EA, Jo S, Preite NZ, Farkas E, Mohacsik P, Fekete C, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015; 100:920–33.
Article59. Jo S, Fonseca TL, Bocco BM, Fernandes GW, McAninch EA, Bolin AP, et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest. 2019; 129:230–45.
Article60. Gumieniak O, Perlstein TS, Williams JS, Hopkins PN, Brown NJ, Raby BA, et al. Ala92 type 2 deiodinase allele increases risk for the development of hypertension. Hypertension. 2007; 49:461–6.
Article61. Mizuma H, Murakami M, Mori M. Thyroid hormone activation in human vascular smooth muscle cells: expression of type II iodothyronine deiodinase. Circ Res. 2001; 88:313–8.62. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014; 94:355–82.
Article63. Wajner SM, Maia AL. New insights toward the acute non-thyroidal illness syndrome. Front Endocrinol (Lausanne). 2012; 3:8.
Article64. Lehnen TE, Santos MV, Lima A, Maia AL, Wajner SM. N-acetylcysteine prevents low T3 syndrome and attenuates cardiac dysfunction in a male rat model of myocardial infarction. Endocrinology. 2017; 158:1502–10.
Article65. Li Q, Qi X, Jia W. 3,3′,5-Triiodothyroxine inhibits apoptosis and oxidative stress by the PKM2/PKM1 ratio during oxygen-glucose deprivation/reperfusion AC16 and HCM-a cells: T3 inhibits apoptosis and oxidative stress by PKM2/PKM1 ratio. Biochem Biophys Res Commun. 2016; 475:51–6.66. von Hafe M, Neves JS, Vale C, Borges-Canha M, Leite-Moreira A. The impact of thyroid hormone dysfunction on ischemic heart disease. Endocr Connect. 2019; 8:R76–90.
Article67. Olivares EL, Marassi MP, Fortunato RS, da Silva AC, Costa-e-Sousa RH, Araujo IG, et al. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology. 2007; 148:4786–92.68. de Castro AL, Tavares AV, Fernandes RO, Campos C, Conzatti A, Siqueira R, et al. T3 and T4 decrease ROS levels and increase endothelial nitric oxide synthase expression in the myocardium of infarcted rats. Mol Cell Biochem. 2015; 408:235–43.
Article69. Bashandy SA, El Awdan SA, Ebaid H, Alhazza IM. Antioxidant potential of spirulina platensis mitigates oxidative stress and reprotoxicity induced by sodium arsenite in male rats. Oxid Med Cell Longev. 2016; 2016:7174351.70. Taki-Eldin A, Zhou L, Xie HY, Chen KJ, Yu D, He Y, et al. Triiodothyronine attenuates hepatic ischemia/reperfusion injury in a partial hepatectomy model through inhibition of proinflammatory cytokines, transcription factors, and adhesion molecules. J Surg Res. 2012; 178:646–56.
Article71. Corssac GB, de Castro AL, Tavares AV, Campos C, Fernandes RO, Ortiz VD, et al. Thyroid hormones effects on oxidative stress and cardiac remodeling in the right ventricle of infarcted rats. Life Sci. 2016; 146:109–16.
Article72. Louzada RA, Carvalho DP. Similarities and differences in the peripheral actions of thyroid hormones and their metabolites. Front Endocrinol (Lausanne). 2018; 9:394.
Article73. Wajner SM, Rohenkohl HC, Serrano T, Maia AL. Sodium selenite supplementation does not fully restore oxidative stress-induced deiodinase dysfunction: implications for the nonthyroidal illness syndrome. Redox Biol. 2015; 6:436–45.
Article74. Didion SP. Cellular and oxidative mechanisms associated with interleukin-6 signaling in the vasculature. Int J Mol Sci. 2017; 18:2563.
Article75. Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007; 9:775–806.
Article76. Valea A, Georgescu CE. Selenoproteins in human body: focus on thyroid pathophysiology. Hormones (Athens). 2018; 17:183–96.
Article77. Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol. 2011; 8:160–71.
Article78. Wang W, Mao J, Zhao J, Lu J, Yan L, Du J, et al. Decreased thyroid peroxidase antibody titer in response to selenium supplementation in autoimmune thyroiditis and the influence of a selenoprotein P gene polymorphism: a prospective, multicenter study in China. Thyroid. 2018; 28:1674–81.
Article79. Mantovani G, Isidori AM, Moretti C, Di Dato C, Greco E, Ciolli P, et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine. 2019; 66:542–50.
Article80. Rostami R, Nourooz-Zadeh S, Mohammadi A, Khalkhali HR, Ferns G, Nourooz-Zadeh J. Serum selenium status and its interrelationship with serum biomarkers of thyroid function and antioxidant defense in Hashimoto’s thyroiditis. Antioxidants (Basel). 2020; 9:1070.
Article81. Marschner RA, Banda P, Wajner SM, Markoski MM, Schaun M, Lehnen AM. Short-term exercise training improves cardiac function associated to a better antioxidant response and lower type 3 iodothyronine deiodinase activity after myocardial infarction. PLoS One. 2019; 14:e0222334.
Article82. Abassi W, Ouerghi N, Ghouili H, Haouami S, Bouassida A. Greater effects of high-compared with moderate-intensity interval training on thyroid hormones in overweight/obese adolescent girls. Horm Mol Biol Clin Investig. 2020; 41:1–7.83. Adamopoulos S, Gouziouta A, Mantzouratou P, Laoutaris ID, Dritsas A, Cokkinos DV, et al. Thyroid hormone signalling is altered in response to physical training in patients with end-stage heart failure and mechanical assist devices: potential physiological consequences? Interact Cardiovasc Thorac Surg. 2013; 17:664–8.
Article84. Hackney AC, Davis HC, Lane AR. Growth hormone-insulin-like growth factor axis, thyroid axis, prolactin, and exercise. Front Horm Res. 2016; 47:1–11.
Article