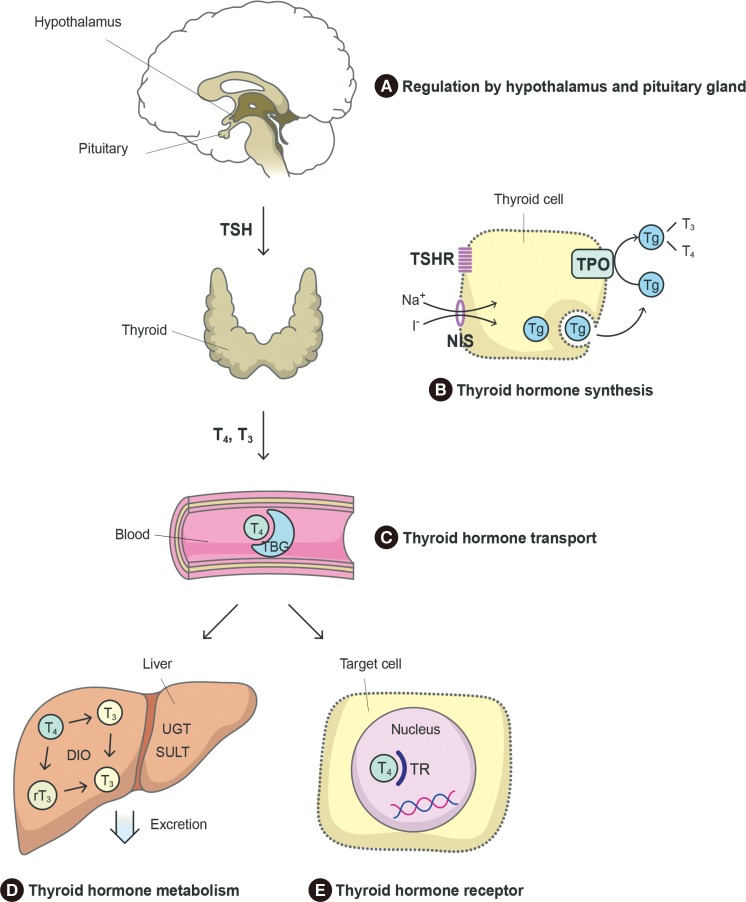
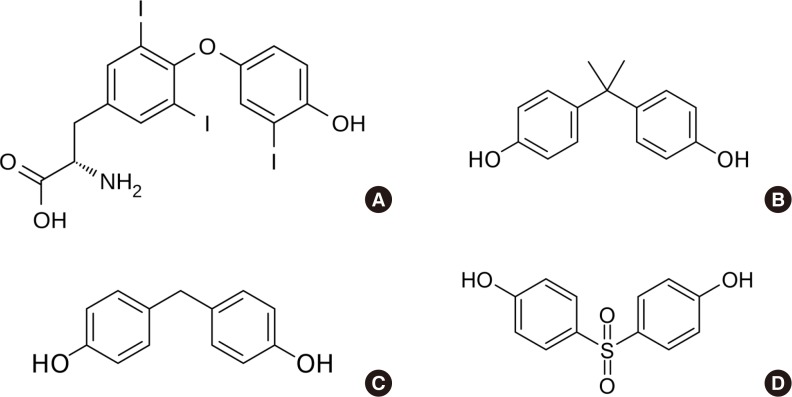
Endocrinol Metab.
2019 Dec;34(4):340-348. 10.3803/EnM.2019.34.4.340.
Bisphenols and Thyroid Hormone
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. yjparkmd@snu.ac.kr
- KMID: 2466252
- DOI: http://doi.org/10.3803/EnM.2019.34.4.340
Abstract
- In recent decades, attention has been directed toward the effects of bisphenol A (BPA) on human health. BPA has estrogenic activity and is regarded as a representative endocrine disruptor. In addition, mounting evidence indicates that BPA can disrupt thyroid hormone and its action. This review examined human epidemiological studies to investigate the association between BPA exposure and thyroid hormone levels, and analyzed in vivo and in vitro experiments to identify the causal relationship and its mechanism of action. BPA is involved in thyroid hormone action not only as a thyroid hormone receptor antagonist, but also through several other mechanisms. Since the use of bisphenols other than BPA has recently increased, we also reviewed the effects of other bisphenols on thyroid hormone action.
MeSH Terms
Figure
Cited by 1 articles
-
Associations of Phthalate Metabolites and Bisphenol A Levels with Obesity in Children: The Korean National Environmental Health Survey (KoNEHS) 2015 to 2017
Moon Young Seo, Shinje Moon, Shin-Hye Kim, Mi Jung Park
Endocrinol Metab. 2022;37(2):249-260. doi: 10.3803/EnM.2021.1235.
Reference
-
1. Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006; 226:79–89. PMID: 16860916.
Article2. Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, et al. Global assessment of bisphenol a in the environment: review and analysis of its occurrence and bioaccumulation. Dose Response. 2015; 13:1559325815598308. PMID: 26674671.3. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008; 116:39–44. PMID: 18197297.4. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Executive summary to EDC-2: the Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015; 36:593–602. PMID: 26414233.
Article5. Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect. 2011; 119:1396–1402. PMID: 21749963.
Article6. Park C, Choi W, Hwang M, Lee Y, Kim S, Yu S, et al. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2012-2014. Sci Total Environ. 2017; 584-585:950–957. PMID: 28153396.7. Sriphrapradang C, Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. Association between bisphenol A and abnormal free thyroxine level in men. Endocrine. 2013; 44:441–447. PMID: 23377699.
Article8. Wang T, Lu J, Xu M, Xu Y, Li M, Liu Y, et al. Urinary bisphenol a concentration and thyroid function in Chinese adults. Epidemiology. 2013; 24:295–302. PMID: 23337242.
Article9. Andrianou XD, Gangler S, Piciu A, Charisiadis P, Zira C, Aristidou K, et al. Human exposures to bisphenol A, bisphenol F and chlorinated bisphenol A derivatives and thyroid function. PLoS One. 2016; 11:e0155237. PMID: 27783680.
Article10. Geens T, Dirtu AC, Dirinck E, Malarvannan G, Van Gaal L, Jorens PG, et al. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ Int. 2015; 76:98–105. PMID: 25575039.
Article11. Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010; 44:1458–1463. PMID: 20030380.
Article12. Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. The association of serum bisphenol A with thyroid autoimmunity. Int J Environ Res Public Health. 2016; 13:E1153. PMID: 27869686.
Article13. Derakhshan A, Shu H, Peeters RP, Kortenkamp A, Lindh CH, Demeneix B, et al. Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environ Int. 2019; 133(Pt A):105123. PMID: 31521814.
Article14. Aung MT, Johns LE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD. Thyroid hormone parameters during pregnancy in relation to urinary bisphenol A concentrations: a repeated measures study. Environ Int. 2017; 104:33–40. PMID: 28410473.
Article15. Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016; 151:30–37. PMID: 27448730.
Article16. Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, et al. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect. 2013; 121:138–144. PMID: 23052180.
Article17. Romano ME, Webster GM, Vuong AM, Thomas Zoeller R, Chen A, Hoofnagle AN, et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: the HOME Study. Environ Res. 2015; 138:453–460. PMID: 25794847.
Article18. Yi B, Kim C, Park M, Han Y, Park JY, Yang M. Association between endocrine disrupting phenols in colostrums and maternal and infant health. Int J Endocrinol. 2013; 2013:282381. PMID: 23737772.
Article19. Minatoya M, Sasaki S, Araki A, Miyashita C, Itoh S, Yamamoto J, et al. Cord blood bisphenol a levels and reproductive and thyroid hormone levels of neonates: the Hokkaido study on environment and childre's health. Epidemiology. 2017; 28 Suppl 1:S3–S9. PMID: 29028670.20. Sanlidag B, Dalkan C, Yetkin O, Bahceciler NN. Evaluation of dose dependent maternal exposure to bisphenol a on thyroid functions in newborns. J Clin Med. 2018; 7:E119. PMID: 29882905.
Article21. Brucker-Davis F, Ferrari P, Boda-Buccino M, Wagner-Mahler K, Pacini P, Gal J, et al. Cord blood thyroid tests in boys born with and without cryptorchidism: correlations with birth parameters and in utero xenobiotics exposure. Thyroid. 2011; 21:1133–1141. PMID: 21875366.22. Teeguarden JG, Waechter JM Jr, Clewell HJ 3rd, Covington TR, Barton HA. Evaluation of oral and intravenous route pharmacokinetics, plasma protein binding, and uterine tissue dose metrics of bisphenol A: a physiologically based pharmacokinetic approach. Toxicol Sci. 2005; 85:823–838. PMID: 15746009.
Article23. Kim S, Kim S, Won S, Choi K. Considering common sources of exposure in association studies: urinary benzophenone-3 and DEHP metabolites are associated with altered thyroid hormone balance in the NHANES 2007-2008. Environ Int. 2017; 107:25–32. PMID: 28651165.24. da Silva MM, Goncalves CFL, Miranda-Alves L, Fortunato RS, Carvalho DP, Ferreira ACF. Inhibition of type 1 iodothyronine deiodinase by bisphenol A. Horm Metab Res. 2019; 51:671–677. PMID: 31174228.
Article25. Fernandez MO, Bourguignon NS, Arocena P, Rosa M, Libertun C, Lux-Lantos V. Neonatal exposure to bisphenol A alters the hypothalamic-pituitary-thyroid axis in female rats. Toxicol Lett. 2018; 285:81–86. PMID: 29305326.
Article26. Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005; 146:607–612. PMID: 15498886.
Article27. Xu X, Liu Y, Sadamatsu M, Tsutsumi S, Akaike M, Ushijima H, et al. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci Res. 2007; 58:149–155. PMID: 17412439.
Article28. Sadowski RN, Park P, Neese SL, Ferguson DC, Schantz SL, Juraska JM. Effects of perinatal bisphenol A exposure during early development on radial arm maze behavior in adult male and female rats. Neurotoxicol Teratol. 2014; 42:17–24. PMID: 24440629.
Article29. Bansal R, Zoeller RT. CLARITY-BPA: bisphenol A or propylthiouracil on thyroid function and effects in the developing male and female rat brain. Endocrinology. 2019; 160:1771–1785. PMID: 31135896.
Article30. Santos-Silva AP, de Moura EG, Pinheiro CR, Oliveira E, Lisboa PC. Short-term and long-term effects of bisphenol A (BPA) exposure during breastfeeding on the biochemical and endocrine profiles in rats. Horm Metab Res. 2018; 50:491–503. PMID: 29883975.
Article31. Kobayashi K, Miyagawa M, Wang RS, Suda M, Sekiguchi S, Honma T. Effects of in utero and lactational exposure to bisphenol A on thyroid status in F1 rat offspring. Ind Health. 2005; 43:685–690. PMID: 16294924.
Article32. Jiang W, Cao L, Wang F, Ge H, Wu PC, Li XW, et al. Accelerated reduction of serum thyroxine and hippocampal histone acetylation links to exacerbation of spatial memory impairment in aged CD-1 mice pubertally exposed to bisphenol-A. Age (Dordr). 2016; 38:405–418. PMID: 27631330.
Article33. Lee S, Kim C, Shin H, Kho Y, Choi K. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere. 2019; 221:115–123. PMID: 30639807.
Article34. Wang N, Zhou Y, Fu C, Wang H, Huang P, Wang B, et al. Influence of bisphenol a on thyroid volume and structure independent of iodine in school children. PLoS One. 2015; 10:e0141248. PMID: 26496713.
Article35. Ahmed RG. Maternal bisphenol A alters fetal endocrine system: thyroid adipokine dysfunction. Food Chem Toxicol. 2016; 95:168–174. PMID: 27326465.
Article36. Silva MMD, Xavier LLF, Goncalves CFL, Santos-Silva AP, Paiva-Melo FD, Freitas ML, et al. Bisphenol A increases hydrogen peroxide generation by thyrocytes both in vivo and in vitro. Endocr Connect. 2018; 7:1196–1207.37. Gentilcore D, Porreca I, Rizzo F, Ganbaatar E, Carchia E, Mallardo M, et al. Bisphenol A interferes with thyroid specific gene expression. Toxicology. 2013; 304:21–31. PMID: 23238275.
Article38. Berto-Junior C, Santos-Silva AP, Ferreira ACF, Graceli JB, de Carvalho DP, Soares P, et al. Unraveling molecular targets of bisphenol A and S in the thyroid gland. Environ Sci Pollut Res Int. 2018; 25:26916–26926. PMID: 30006815.39. Lee S, Kim C, Youn H, Choi K. Thyroid hormone disrupting potentials of bisphenol A and its analogues: in vitro comparison study employing rat pituitary (GH3) and thyroid follicular (FRTL-5) cells. Toxicol In Vitro. 2017; 40:297–304. PMID: 28167136.40. Wu Y, Beland FA, Fang JL. Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol In Vitro. 2016; 32:310–319. PMID: 26827900.
Article41. Dong H, Wade MG. Application of a nonradioactive assay for high throughput screening for inhibition of thyroid hormone uptake via the transmembrane transporter MCT8. Toxicol In Vitro. 2017; 40:234–242. PMID: 28119167.
Article42. Cao J, Guo LH, Wan B, Wei Y. In vitro fluorescence displacement investigation of thyroxine transport disruption by bisphenol A. J Environ Sci (China). 2011; 23:315–321. PMID: 21517007.
Article43. Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, et al. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 2008; 232:150–160. PMID: 18647617.
Article44. Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002; 87:5185–5190. PMID: 12414890.
Article45. Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro. 2011; 25:257–266. PMID: 20732405.
Article46. Sheng ZG, Tang Y, Liu YX, Yuan Y, Zhao BQ, Chao XJ, et al. Low concentrations of bisphenol a suppress thyroid hormone receptor transcription through a nongenomic mechanism. Toxicol Appl Pharmacol. 2012; 259:133–142. PMID: 22227104.
Article47. Heimeier RA, Das B, Buchholz DR, Shi YB. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology. 2009; 150:2964–2973. PMID: 19228888.
Article48. Lu L, Zhan T, Ma M, Xu C, Wang J, Zhang C, et al. Thyroid disruption by bisphenol S Analogues via thyroid hormone receptor β: in vitro, in vivo, and molecular dynamics simulation study. Environ Sci Technol. 2018; 52:6617–6625. PMID: 29763311.
Article49. Zhang YF, Ren XM, Li YY, Yao XF, Li CH, Qin ZF, et al. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo. Environ Pollut. 2018; 237:1072–1079. PMID: 29146198.50. Huang GM, Tian XF, Fang XD, Ji FJ. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere. 2016; 147:188–194. PMID: 26766355.
Article51. Zhang DH, Zhou EX, Yang ZL. Waterborne exposure to BPS causes thyroid endocrine disruption in zebrafish larvae. PLoS One. 2017; 12:e0176927. PMID: 28467477.
Article52. Naderi M, Wong MY, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol. 2014; 148:195–203. PMID: 24508763.
Article53. Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Calafat AM, et al. A repeated measures study of phenol, paraben and triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ Health. 2019; 18:28. PMID: 30940137.
Article54. Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: a repeated measures study. Environ Int. 2018; 113:341–349. PMID: 29366524.
Article55. Zhou Z, Zhang J, Jiang F, Xie Y, Zhang X, Jiang L. Higher urinary bisphenol A concentration and excessive iodine intake are associated with nodular goiter and papillary thyroid carcinoma. Biosci Rep. 2017; 37:BSR20170678. PMID: 28684549.
Article56. Li L, Ying Y, Zhang C, Wang W, Li Y, Feng Y, et al. Bisphenol A exposure and risk of thyroid nodules in Chinese women: a case-control study. Environ Int. 2019; 126:321–328. PMID: 30825751.
Article57. Takagi H, Mitsumori K, Onodera H, Nasu M, Tamura T, Yasuhara K, et al. Improvement of a two-stage carcinogenesis model to detect modifying effects of endocrine disrupting chemicals on thyroid carcinogenesis in rats. Cancer Lett. 2002; 178:1–9. PMID: 11849735.
Article58. Zhang J, Zhang X, Li Y, Zhou Z, Wu C, Liu Z, et al. Low dose of bisphenol A enhance the susceptibility of thyroid carcinoma stimulated by DHPN and iodine excess in F344 rats. Oncotarget. 2017; 8:69874–69887. PMID: 29050248.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resistance to thyroid hormone due to a novel mutation of thyroid hormone receptor beta gene
- The Effect of Thyroid Hormone Deficiensy on Growth Hormone Levels
- Insulin Resistance and Intracellular Thyroid Hormone Dysfunction
- Monogenic Thyroid Disorder
- A Case of Resistance Syndrome to Thyroid Hormone Associated with Mutation (G345D) in the Thyroid Hormone Receptor Beta Gene