Cancer Res Treat.
2021 Oct;53(4):1156-1165. 10.4143/crt.2020.1356.
Neoadjuvant Chemotherapy–Guided Bladder-Sparing Treatment for Muscle-Invasive Bladder Cancer: Results of a Pilot Phase II Study
- Affiliations
-
- 1Department of Urology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Urology, Cancer Hospital of Huanxing Chaoyang District Beijing, Beijing, China
- 4Department of Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 6Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- KMID: 2521590
- DOI: http://doi.org/10.4143/crt.2020.1356
Abstract
- Purpose
Reduced quality of life after cystectomy has made bladder preservation a popular research topic for muscle-invasive bladder cancer (MIBC). Previous research has indicated significant tumor downstaging after neoadjuvant chemotherapy (NAC). However, maximal transurethral resection of bladder tumor (TURBT) was performed before NAC to define the pathology, impacting the real evaluation of NAC. This research aimed to assess real NAC efficacy without interference from TURBT and apply combined modality therapies guided by NAC efficacy.
Materials and Methods
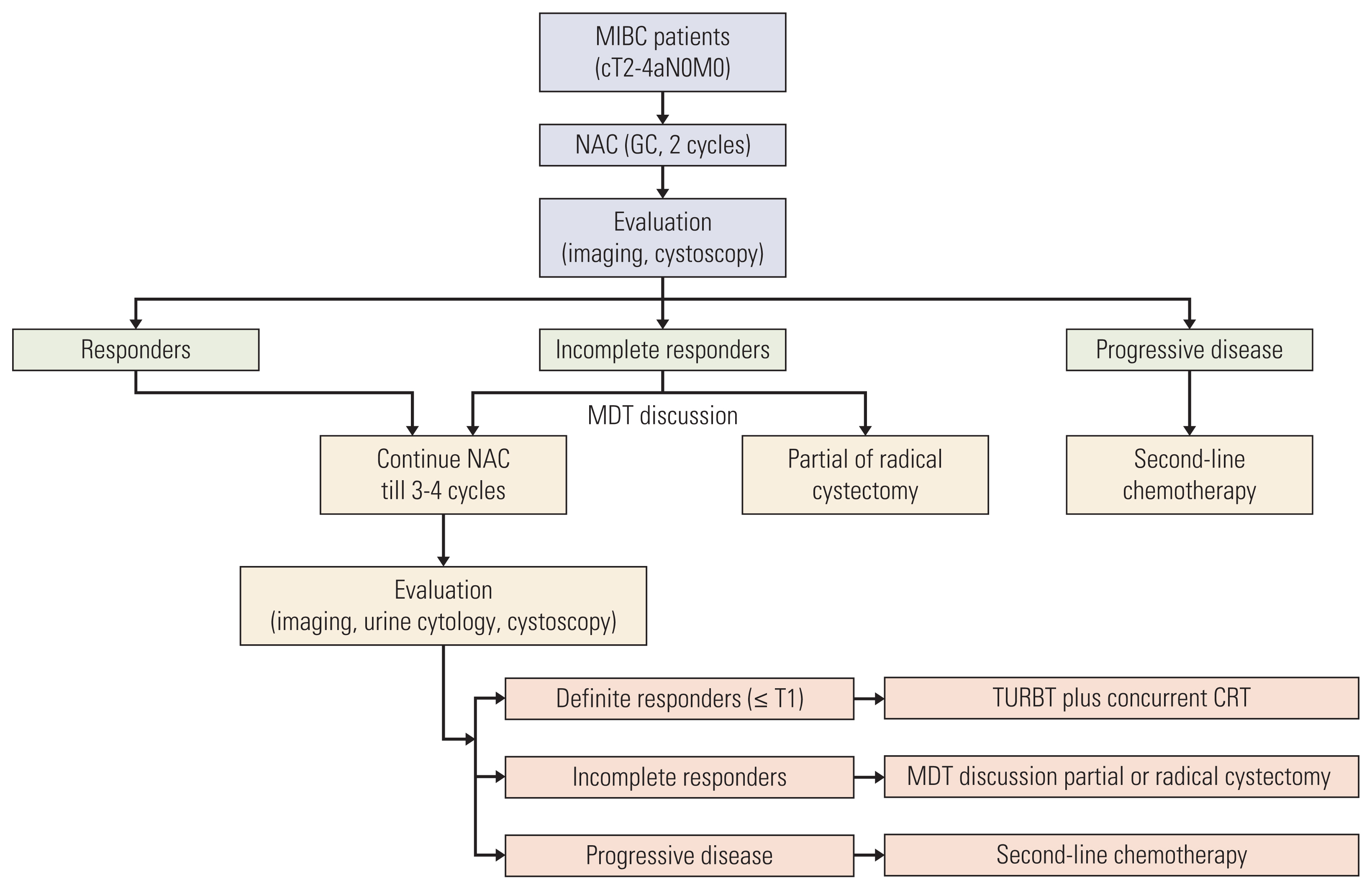
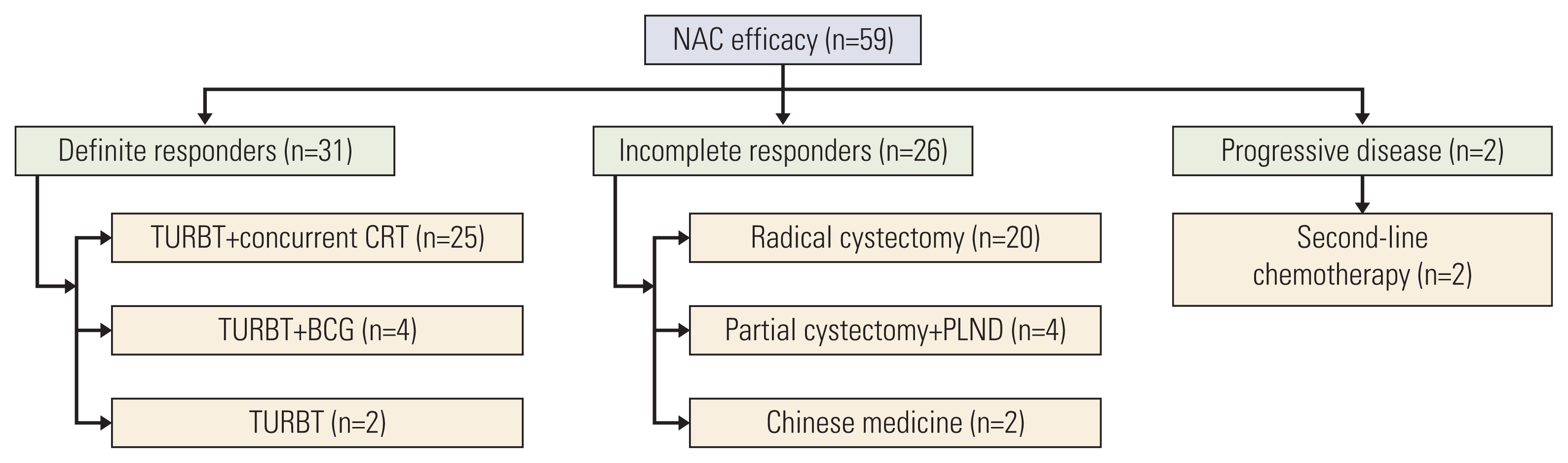
Patients with cT2-4aN0M0 MIBC were confirmed by cystoscopic biopsy and imaging. NAC efficacy was assessed by imaging, urine cytology, and cystoscopy with multidisciplinary team discussion. Definite responders (≤ T1) underwent TURBT plus concurrent chemoradiotherapy. Incomplete responders underwent radical cystectomy or partial cystectomy if feasible. The primary endpoint was the bladder preservation rate.
Results
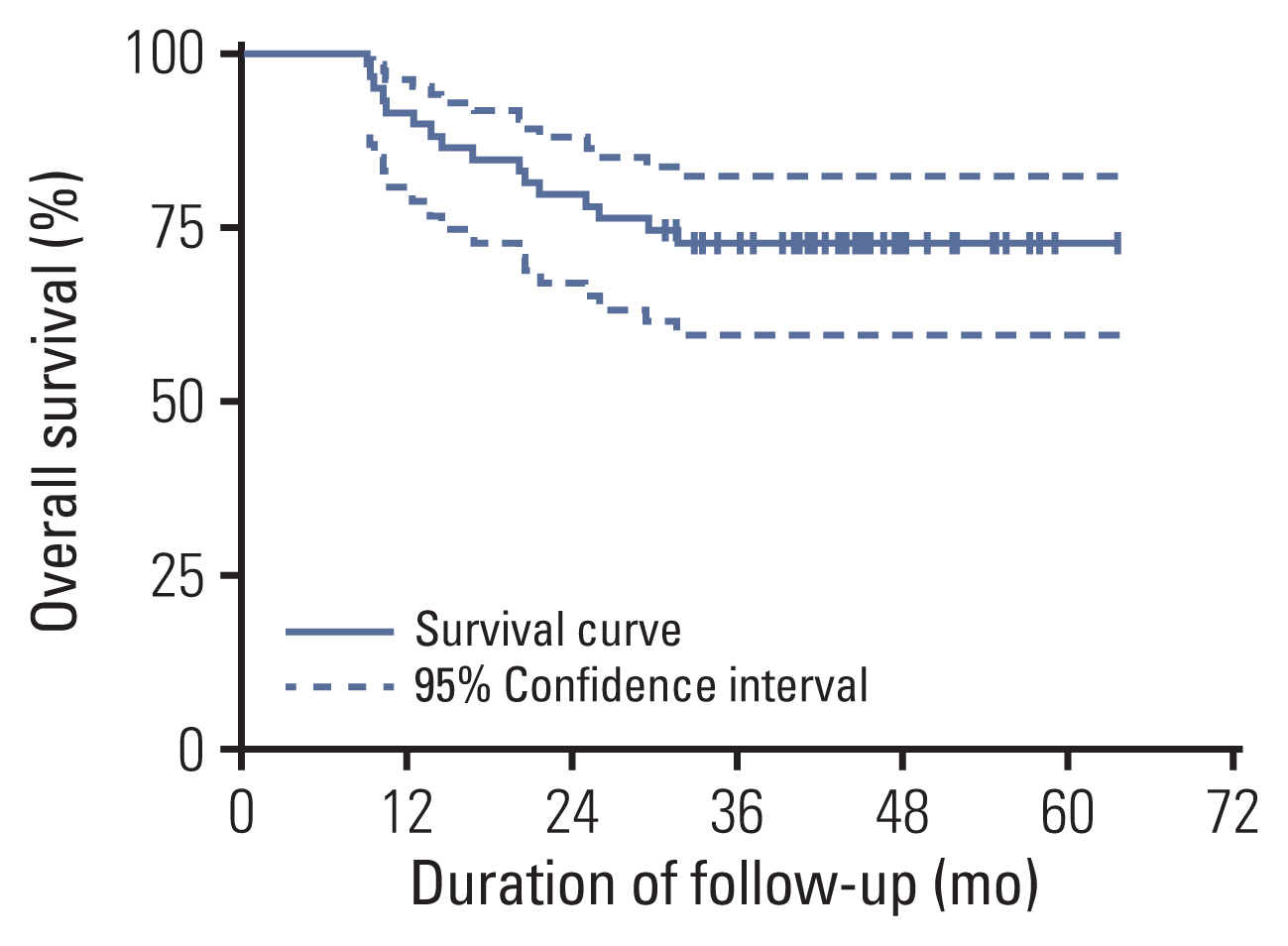
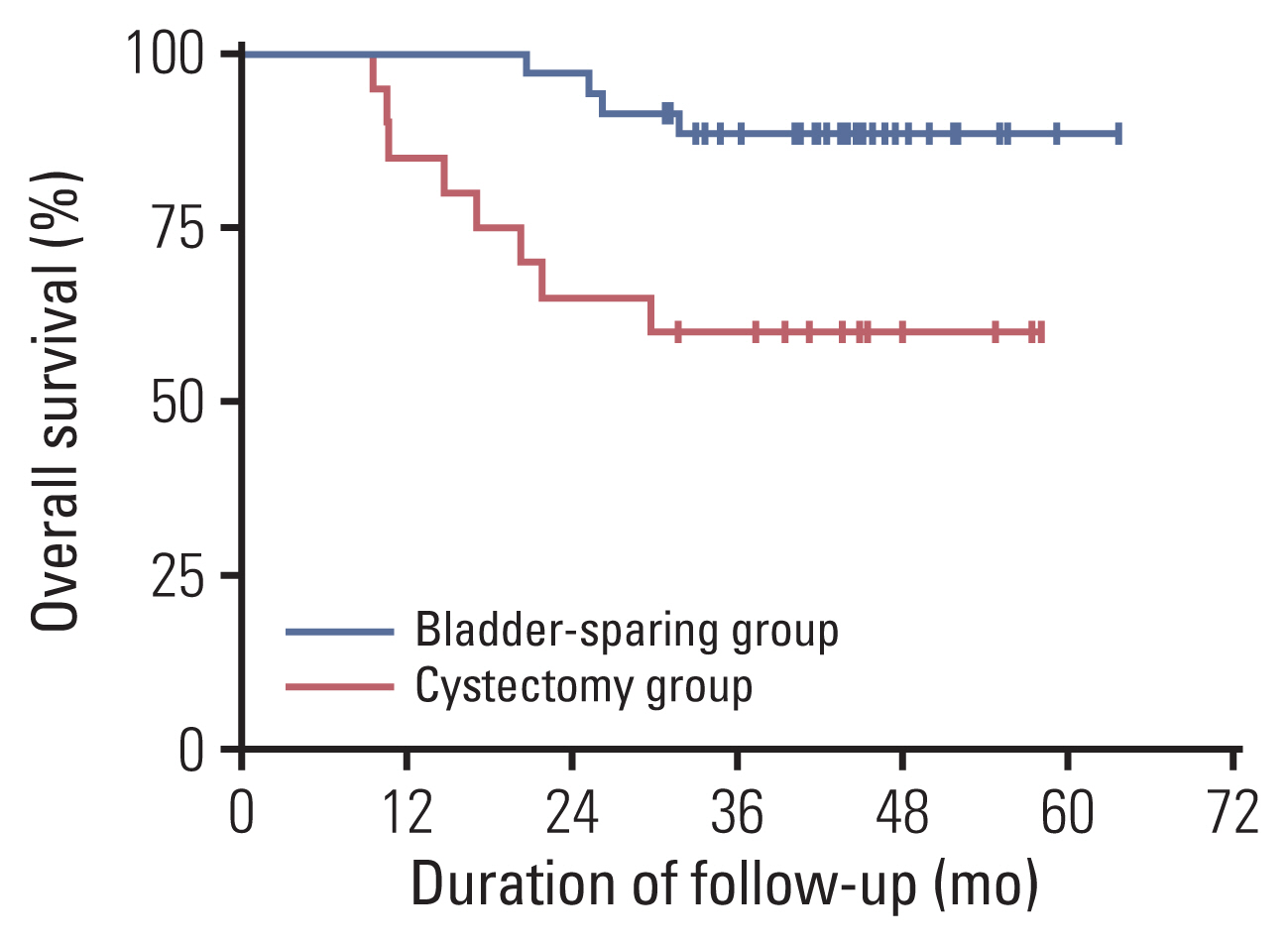
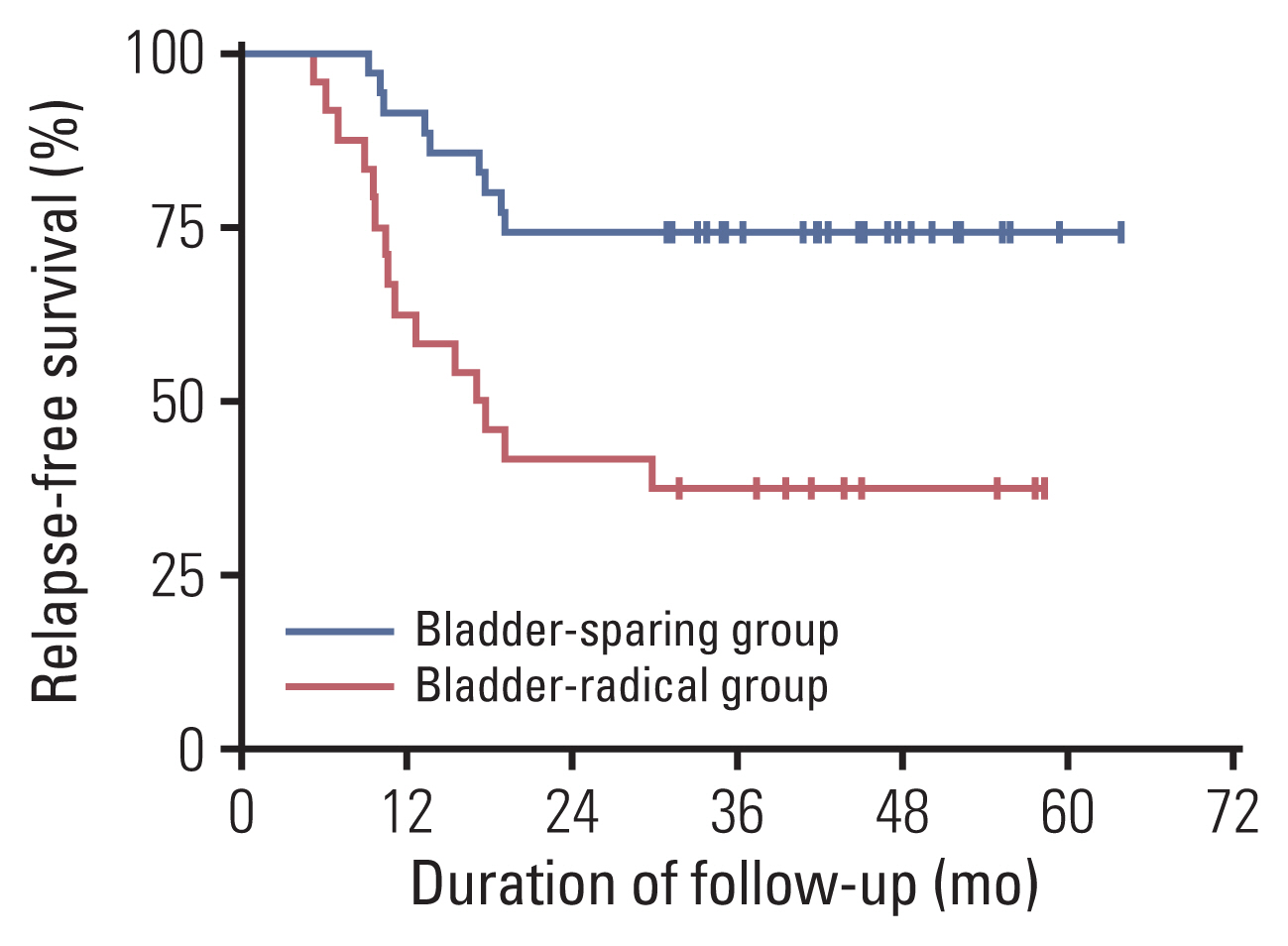
Fifty-nine patients were enrolled, and the median age was 63 years. Patients with cT3-4 accounted for 75%. The median number of NAC cycles was three. Definite responders were 52.5%. The complete response (CR) was 10.2%, and 59.3% of patients received bladder-sparing treatments. With a median follow-up of 44.6 months, the 3-year overall survival (OS) was 72.8%. Three-year OS and relapse-free survival were 88.4% and 60.0% in the bladder-sparing group but only 74.3% and 37.5% in the cystectomy group. The evaluations of preserved bladder function were satisfactory.
Conclusion
After stratifying MIBC patients by NAC efficacy, definite responders achieved a satisfactory bladder-sparing rate, prognosis, and bladder function. The CR rate reflected the real NAC efficacy for MIBC. This therapy is worth verifying through multicenter research.
Keyword
Figure
Reference
-
References
1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017; 71:96–108.
Article2. Flaig TW. NCCN guidelines updates: management of muscle-invasive bladder cancer. J Natl Compr Canc Netw. 2019; 17:591–3.3. International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party; European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011; 29:2171–7.4. Parekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018; 391:2525–36.5. Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, et al. Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol. 2012; 61:1239–44.
Article6. Winters BR, Wright JL, Holt SK, Dash A, Gore JL, Schade GR. Health related quality of life following radical cystectomy: comparative analysis from the medicare health outcomes survey. J Urol. 2018; 199:669–75.
Article7. Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017; 35:2299–305.
Article8. Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012; 61:705–11.
Article9. Giacalone NJ, Shipley WU, Clayman RH, Niemierko A, Drumm M, Heney NM, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the Massachusetts General Hospital experience. Eur Urol. 2017; 71:952–60.
Article10. Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003; 349:859–66.
Article11. Yeshchina O, Badalato GM, Wosnitzer MS, Hruby G, RoyChoudhury A, Benson MC, et al. Relative efficacy of perioperative gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. 2012; 79:384–90.
Article12. Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015; 121:2586–93.
Article13. Lee FC, Harris W, Cheng HH, Shenoi J, Zhao S, Wang J, et al. Pathologic response rates of gemcitabine/cisplatin versus methotrexate/vinblastine/adriamycin/cisplatin neoadjuvant chemotherapy for muscle invasive urothelial bladder cancer. Adv Urol. 2013; 2013:317190.
Article14. Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018; 4:1535–42.
Article15. Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009; 55:815–25.
Article16. van der Pol CB, Chung A, Lim C, Gandhi N, Tu W, McInnes MD, et al. Update on multiparametric MRI of urinary bladder cancer. J Magn Reson Imaging. 2018; 48:882–96.
Article17. Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging: accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009; 251:112–21.18. Donaldson SB, Bonington SC, Kershaw LE, Cowan R, Lyons J, Elliott T, et al. Dynamic contrast-enhanced MRI in patients with muscle-invasive transitional cell carcinoma of the bladder can distinguish between residual tumour and post-chemotherapy effect. Eur J Radiol. 2013; 82:2161–8.
Article19. Shipley WU, Winter KA, Kaufman DS, Lee WR, Heney NM, Tester WR, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89–03. J Clin Oncol. 1998; 16:3576–83.
Article20. Mathieu R, Lucca I, Klatte T, Babjuk M, Shariat SF. Trimodal therapy for invasive bladder cancer: is it really equal to radical cystectomy? Curr Opin Urol. 2015; 25:476–82.21. Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rodel CM, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014; 66:120–37.
Article22. Zapatero A, Martin De Vidales C, Arellano R, Ibanez Y, Bocardo G, Perez M, et al. Long-term results of two prospective bladder-sparing trimodality approaches for invasive bladder cancer: neoadjuvant chemotherapy and concurrent radio-chemotherapy. Urology. 2012; 80:1056–62.
Article23. Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol. 2012; 61:1039–47.
Article24. Massari F, Santoni M, di Nunno V, Cheng L, Lopez-Beltran A, Cimadamore A, et al. Adjuvant and neoadjuvant approaches for urothelial cancer: updated indications and controversies. Cancer Treat Rev. 2018; 68:80–5.
Article25. Panebianco V, Barchetti F, de Haas RJ, Pearson RA, Kennish SJ, Giannarini G, et al. Improving staging in bladder cancer: the increasing role of multiparametric magnetic resonance imaging. Eur Urol Focus. 2016; 2:113–21.
Article26. Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol. 2018; 74:294–306.27. Zhang M, Chen Y, Cong X, Zhao X. Utility of intravoxel incoherent motion MRI derived parameters for prediction of aggressiveness in urothelial bladder carcinoma. J Magn Reson Imaging. 2018; 48:1648–56.
Article28. Inoue M, Ishioka J, Fukuda H, Kageyama Y, Saito Y, Higashi Y. Clinical outcome of chemoradiotherapy for T1G3 bladder cancer. Int J Urol. 2008; 15:747–50.
Article29. Meleis L, Moore R, Inman BA, Harrison MR. Retrospective analysis of the efficacy and safety of neoadjuvant gemcitabine and cisplatin in muscle-invasive bladder cancer. J Oncol Pharm Pract. 2020; 26:330–7.
Article30. Huddart RA, Birtle A, Maynard L, Beresford M, Blazeby J, Donovan J, et al. Clinical and patient-reported outcomes of SPARE: a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. 2017; 120:639–50.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of immediate neoadjuvant electromotive instillation of mitomycin C with Bacillus Calmette–Guérin versus BCG alone in non-muscle-invasive bladder cancer: A randomized controlled trial
- Neoadjuvant chemotherapy with gemcitabine and cisplatin followed by selective bladder preservation chemoradiotherapy in muscle-invasive urothelial carcinoma of bladder
- Trimodal Therapy in the Treatment of Muscle-Invasive Bladder Cancer
- Accuracy of Vesical Imaging-Reporting and Data System for muscle-invasive bladder cancer detection from multiparametric magnetic resonance imaging
- Perioperative systemic therapy in muscle invasive bladder cancer: Current standard method, biomarkers and emerging strategies