Cancer Res Treat.
2021 Oct;53(4):1134-1147. 10.4143/crt.2020.1191.
Neurocognitive Effects of Chemotherapy for Colorectal Cancer: A Systematic Review and a Meta-Analysis of 11 Studies
- Affiliations
-
- 1Yonsei University College of Medicine, Seoul, Korea
- 2Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Public Health, Yonsei University, Seoul, Korea
- 4Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA, USA
- KMID: 2521588
- DOI: http://doi.org/10.4143/crt.2020.1191
Abstract
- Purpose
Chemotherapy-related cognitive impairment (CRCI) is a controversial concept not much explored on colorectal cancer patients.
Materials and Methods
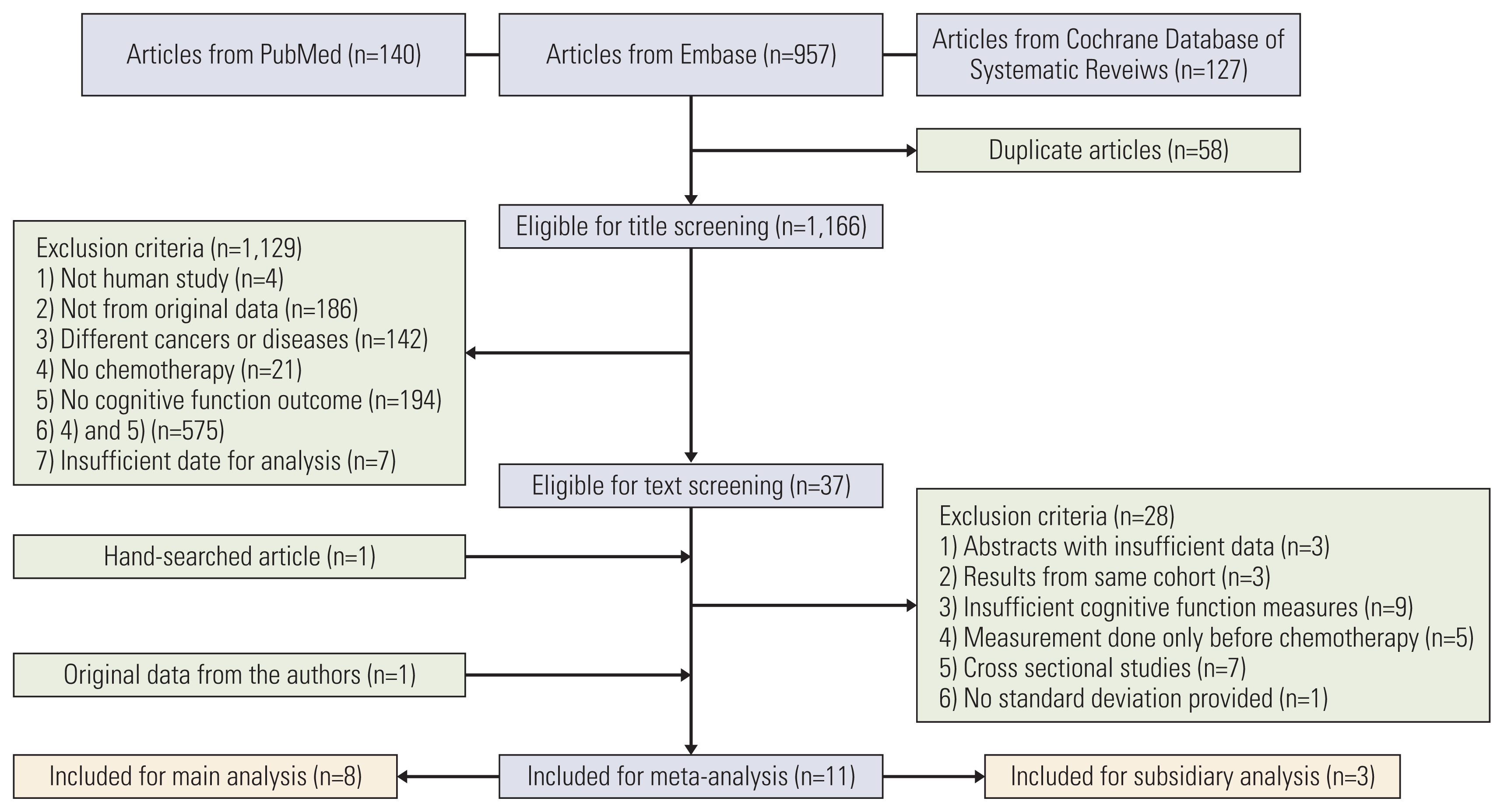
We identified 11 prospective studies: eight studies on 696 colorectal cancer patients who received chemotherapy and three studies on 346 rectal cancer patients who received neoadjuvant chemoradiotherapy. Standardized mean differences (SMDs) of neuropsychological test results and the cognitive quality-of-life scale were calculated using random effect models. A meta-regression was conducted to investigate the association between mean study population age and effect sizes.
Results
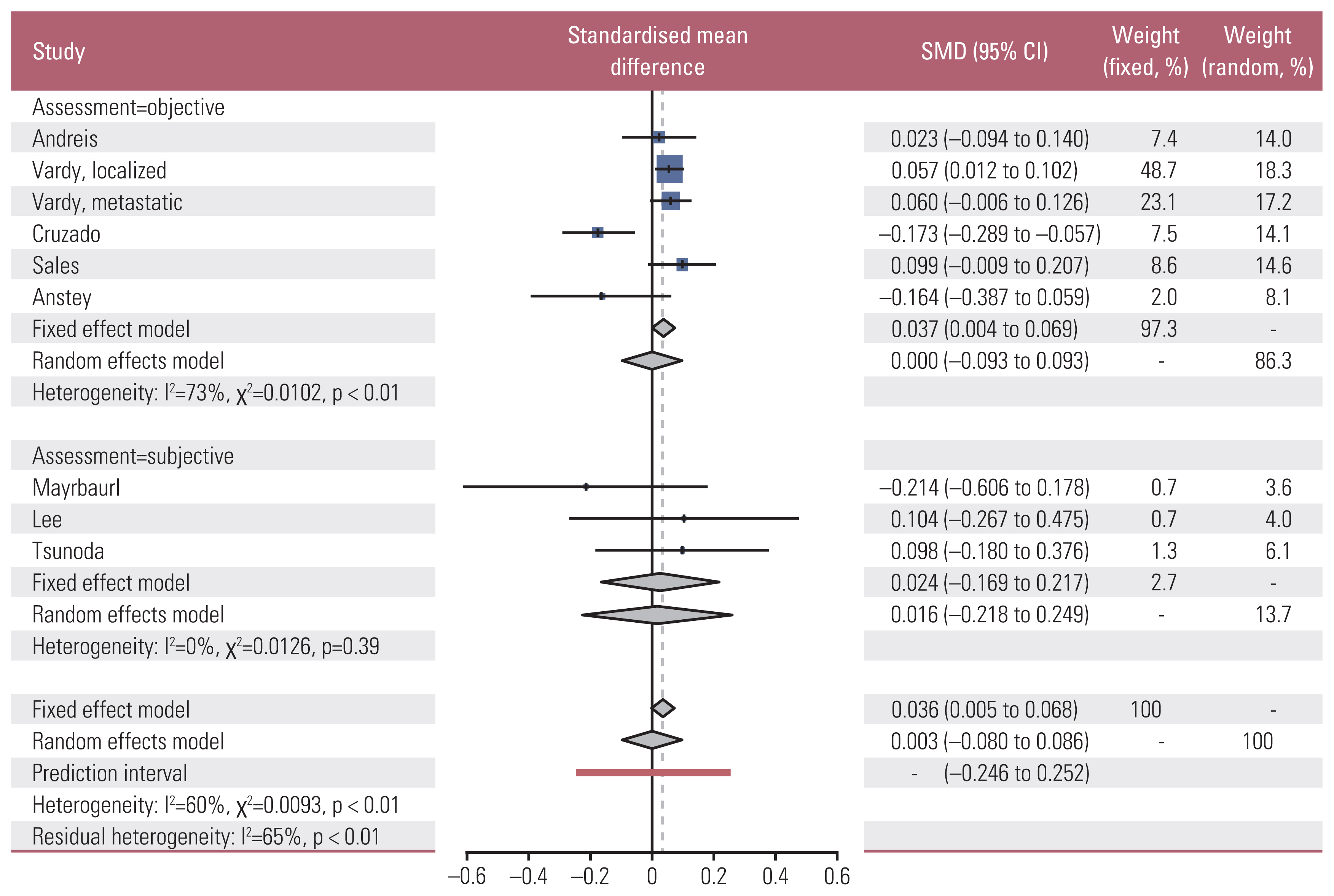
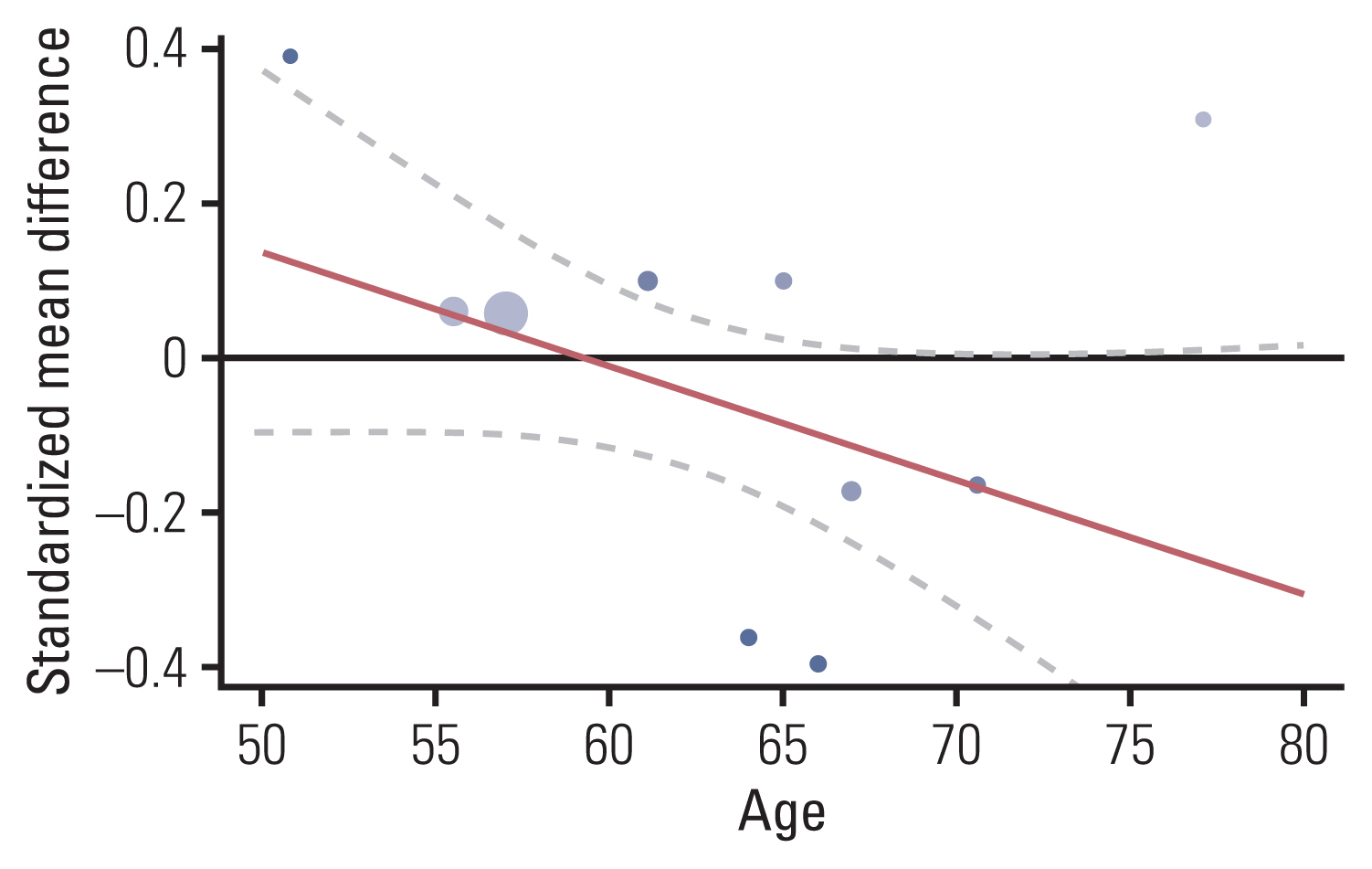
The association between chemotherapy and cognitive impairment was not clear in colorectal cancer patients (SMD, 0.003; 95% confidence interval, ‒0.080 to 0.086). However, a meta-regression showed that older patients are more vulnerable to CRCI than younger patients (β=‒0.016, p < 0.001).
Conclusion
Chemotherapy has an overall positive negligible effect size on the cognitive function of colorectal patients. Age is a significant moderator of CRCI.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998; 90:210–8.
Article3. Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003; 9:967–82.
Article4. Cruzado JA, Lopez-Santiago S, Martinez-Marin V, Jose-Moreno G, Custodio AB, Feliu J. Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support Care Cancer. 2014; 22:1815–23.
Article5. Vardy JL, Dhillon HM, Pond GR, Rourke SB, Bekele T, Renton C, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015; 33:4085–92.
Article6. Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011; 35:729–41.
Article7. Branca JJ, Maresca M, Morucci G, Becatti M, Paternostro F, Gulisano M, et al. Oxaliplatin-induced blood brain barrier loosening: a new point of view on chemotherapy-induced neurotoxicity. Oncotarget. 2018; 9:23426–38.
Article8. Wigmore PM, Mustafa S, El-Beltagy M, Lyons L, Umka J, Bennett G. Effects of 5-FU. Adv Exp Med Biol. 2010; 678:157–64.
Article9. Vardy JL, Bray VJ, Dhillon HM. Cancer-induced cognitive impairment: practical solutions to reduce and manage the challenge. Future Oncol. 2017; 13:767–71.
Article10. Fernandes HA, Richard NM, Edelstein K. Cognitive rehabilitation for cancer-related cognitive dysfunction: a systematic review. Support Care Cancer. 2019; 27:3253–79.
Article11. Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010; 28:4434–40.
Article12. Bleecker ML, Ford DP, Celio MA, Vaughan CG, Lindgren KN. Impact of cognitive reserve on the relationship of lead exposure and neurobehavioral performance. Neurology. 2007; 69:470–6.
Article13. Anstey KJ, Sargent-Cox K, Cherbuin N, Sachdev PS. Self-reported history of chemotherapy and cognitive decline in adults aged 60 and older: the PATH Through Life Project. J Gerontol A Biol Sci Med Sci. 2015; 70:729–35.
Article14. Lee SH, Lee TG, Baek MJ, Kim JJ, Park SS, Lee SJ. Quality of life changes during adjuvant chemotherapy in patients with colon cancer. Korean J Clin Oncol. 2016; 12:60–6.
Article15. Andreis F, Ferri M, Mazzocchi M, Meriggi F, Rizzi A, Rota L, et al. Lack of a chemobrain effect for adjuvant FOLFOX chemotherapy in colon cancer patients: a pilot study. Support Care Cancer. 2013; 21:583–90.
Article16. Sales MV, Suemoto CK, Apolinario D, Serrao V, Andrade CS, Conceicao DM, et al. Effects of adjuvant chemotherapy on cognitive function of patients eith rarly-stage volorectal vancer. Clin Colorectal Cancer. 2019; 18:19–27.17. Mayrbaurl B, Giesinger JM, Burgstaller S, Piringer G, Holzner B, Thaler J. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Support Care Cancer. 2016; 24:667–74.
Article18. Tsunoda A, Nakao K, Watanabe M, Matsui N, Tsunoda Y. Health-related quality of life in patients with colorectal cancer who receive oral uracil and tegafur plus leucovorin. Jpn J Clin Oncol. 2010; 40:412–9.
Article19. Couwenberg AM, Burbach JP, van Grevenstein WM, Smits AB, Consten EC, Schiphorst AH, et al. Effect of neoadjuvant therapy and rectal surgery on health-related quality of life in patients with rectal cancer during the first 2 years after diagnosis. Clin Colorectal Cancer. 2018; 17:e499–512.
Article20. Souza J, Nahas CS, Nahas SC, Marques CF, Ribeiro U Junior, Cecconello I. Health-related quality of life assessment in patients with rectal cancer treated with curative intent. Arq Gastroenterol. 2018; 55:154–9.
Article21. Bencova V, Krajcovicova I, Svec J. Indication of pre-surgical radiochemotherapy enhances psychosocial morbidity among patients with resectable locally advanced rectal cancer. Neoplasma. 2016; 63:635–41.
Article22. Horowitz TS, Trevino M, Gooch IM, Duffy KA. Understanding the profile of cancer-related cognitive impairments: a critique of meta-analyses. J Natl Cancer Inst. 2019; 111:1009–15.
Article23. Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer;2001.24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
Article25. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa, ON: Ottawa Hospital Research Institute;2021. [cited 2021 Feb 9]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .26. Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017; 5:80–4.
Article27. Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008; 33:700–11.28. Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013; 39:297–304.
Article29. Bernstein LJ, McCreath GA, Komeylian Z, Rich JB. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis. Neurosci Biobehav Rev. 2017; 83:417–28.
Article30. Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005; 104:2222–33.
Article31. Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn. 2005; 59:60–70.
Article32. Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006; 20:76–89.
Article33. Ono M, Ogilvie JM, Wilson JS, Green HJ, Chambers SK, Ownsworth T, et al. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol. 2015; 5:59.
Article34. Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012; 30:3578–87.
Article35. Lindner OC, Phillips B, McCabe MG, Mayes A, Wearden A, Varese F, et al. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology. 2014; 28:726–40.
Article36. Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010; 11:118.
Article37. Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological assessment. 4th ed. New York: Oxford University Press;2004.38. McCaffrey RJ, Duff K, Westervelt HJ. Practitioner’s guide to evaluating change with neuropsychological assessment instruments. New York: Springer Science & Business Media;2000.39. McCaffrey RJ, Westervelt HJ. Issues associated with repeated neuropsychological assessments. Neuropsychol Rev. 1995; 5:203–21.
Article40. Dodge HH, Zhu J, Lee CW, Chang CC, Ganguli M. Cohort effects in age-associated cognitive trajectories. J Gerontol A Biol Sci Med Sci. 2014; 69:687–94.
Article41. Dodge HH, Zhu J, Hughes TF, Snitz BE, Chang CH, Jacobsen EP, et al. Cohort effects in verbal memory function and practice effects: a population-based study. Int Psychogeriatr. 2017; 29:137–48.
Article42. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011; 12:703–8.
Article43. Pendergrass JC, Targum SD, Harrison JE. Cognitive impairment associated with cancer: a brief review. Innov Clin Neurosci. 2018; 15:36–44.44. Hardy SJ, Krull KR, Wefel JS, Janelsins M. Cognitive changes in cancer survivors. Am Soc Clin Oncol Educ Book. 2018; 38:795–806.
Article45. Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med. 2015; 13:195.
Article46. Bianchi E, Di Cesare Mannelli L, Micheli L, Farzad M, Agliano M, Ghelardini C. Apoptotic process induced by oxaliplatin in rat hippocampus causes memory impairment. Basic Clin Pharmacol Toxicol. 2017; 120:14–21.
Article47. Fardell JE, Vardy J, Shah JD, Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology (Berl). 2012; 220:183–93.
Article48. Vega JN, Dumas J, Newhouse PA. Cognitive effects of chemotherapy and cancer-related treatments in older adults. Am J Geriatr Psychiatry. 2017; 25:1415–26.
Article49. Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006; 67:212–5.
Article50. Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007; 7:192–201.
Article51. Dikmen SS, Heaton RK, Grant I, Temkin NR. Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. J Int Neuropsychol Soc. 1999; 5:346–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Introduction of the Systematic Review and Meta-Analysis
- Systematic review and meta-analysis of cancer risks in relation to environmental waste incinerator emissions: a meta-analysis of case-control and cohort studies
- Critical Appraisal of Systematic Review/Meta-analysis
- Introduction to systematic review and meta-analysis
- How to review and assess a systematic review and meta-analysis article: a methodological study (secondary publication)