Korean J Physiol Pharmacol.
2021 Nov;25(6):555-564. 10.4196/kjpp.2021.25.6.555.
Beneficial effects of naringenin and morin on interleukin-5 and reactive oxygen species production in BALB/c mice with ovalbumin-induced asthma
- Affiliations
-
- 1Department of Respiratory and Critical Care Treatment, Weifang Wei 'en Hospital, Weifang, Shandong 261031, China
- KMID: 2521481
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.555
Abstract
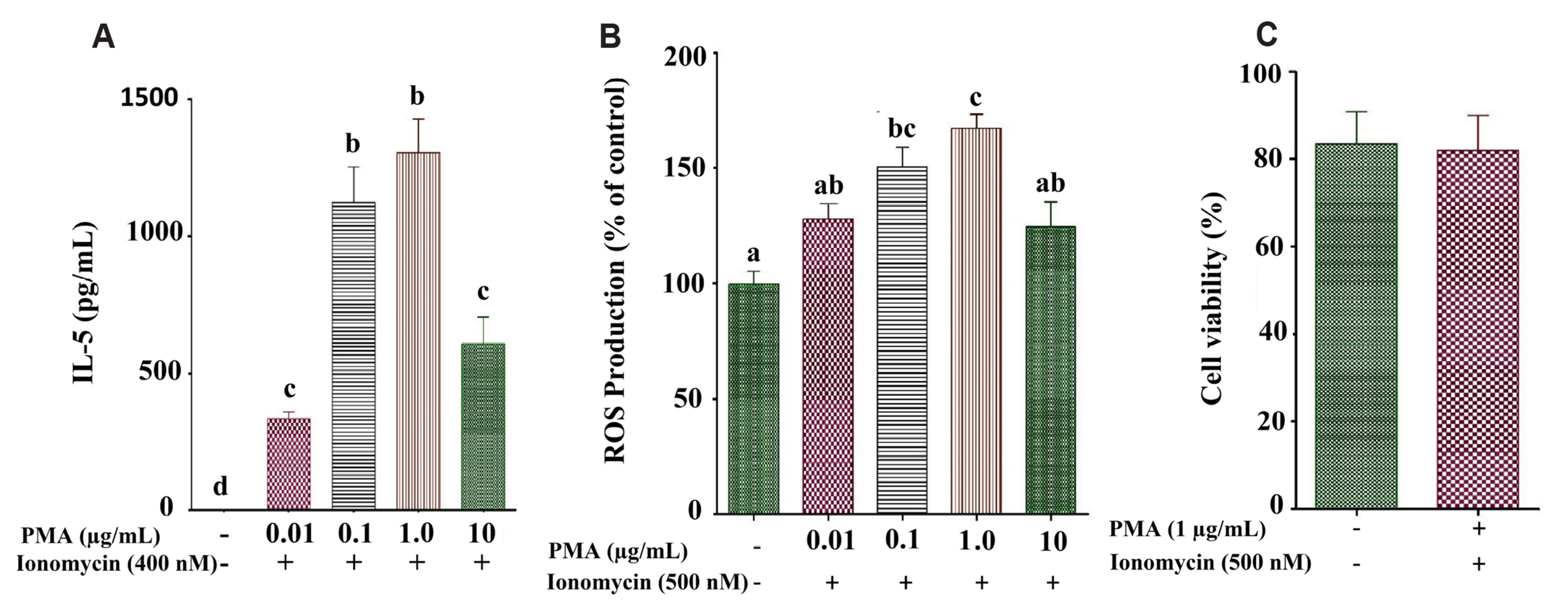
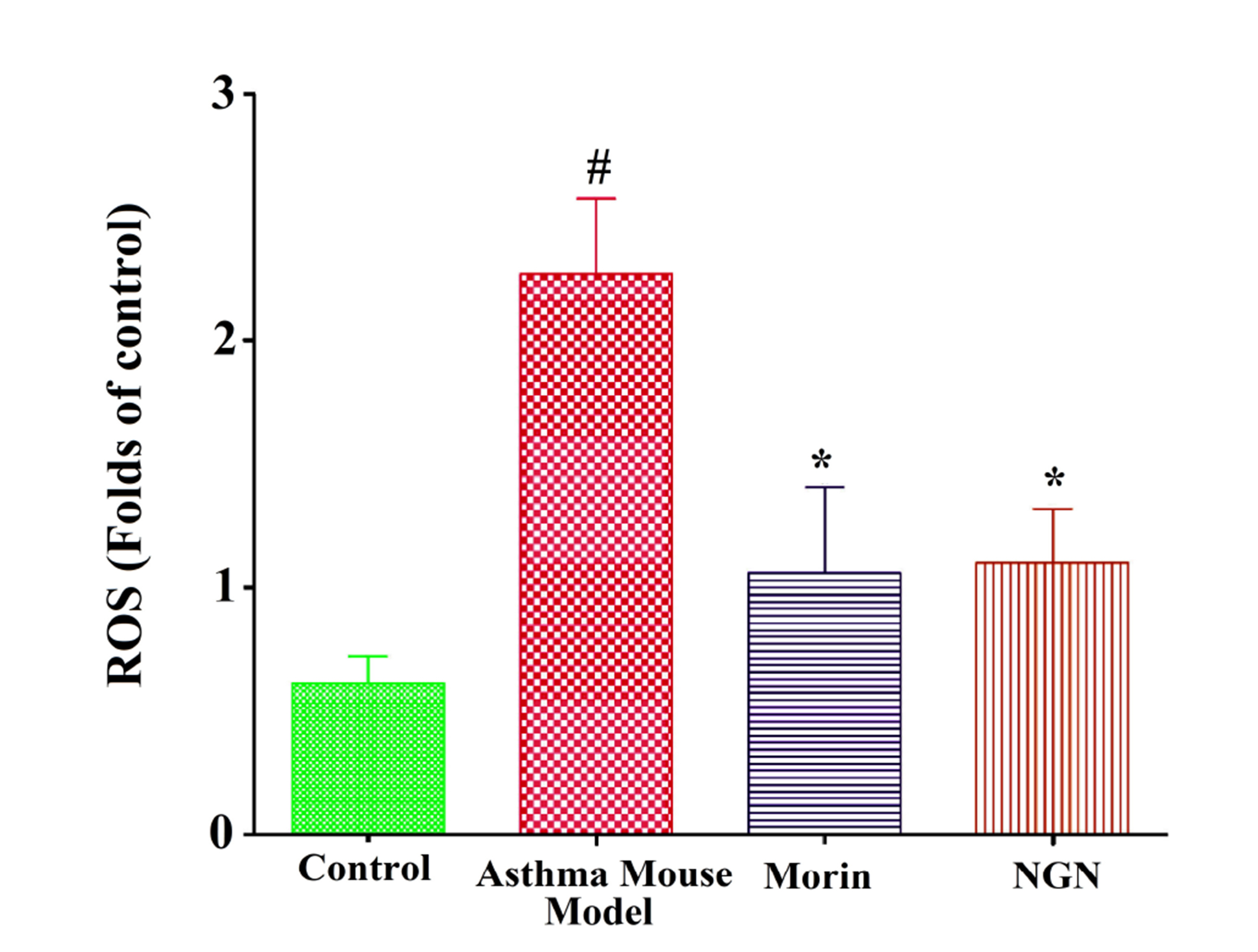
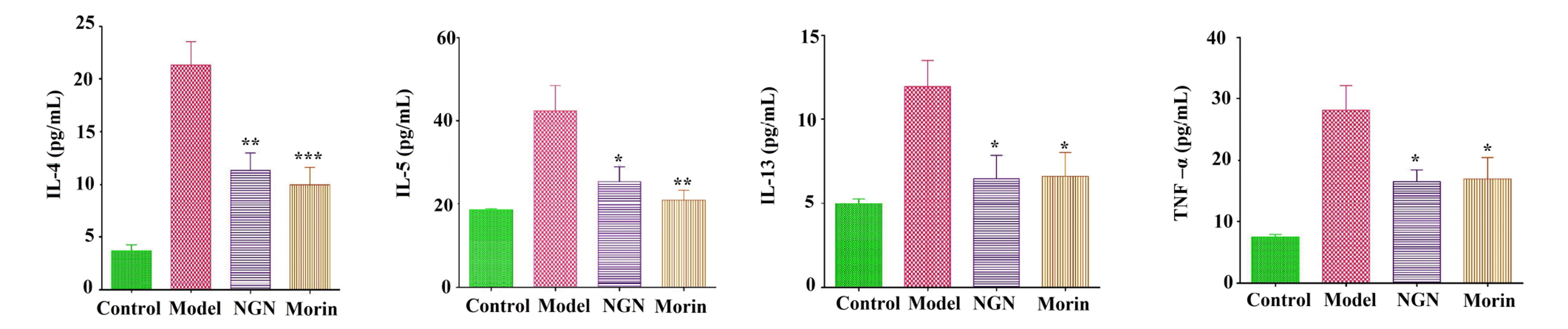
- We investigated the effects of naringenin and morin on IL-5 and ROS production in PMA+ionomycin-treated EL-4 cells with the corroboration of their antioxidant and anti-inflammatory properties using an asthma-induced mouse model. The EL-4 cell line was used to study the outcomes of naringenin or morin, followed by cell viability studies. Western blot analysis and ELISA test were used to determine Th2 mediated cytokines. In vivo studies were carried out on BALB/c mice to induce allergic asthma using ovalbumin administered intraperitoneally. Intracellular ROS was determined using 2’,7’-dichlorodihydrofluorescein diacetate, followed by serum enzymatic (AST and ALT) estimations and inflammatory cell count in the bronchoalveolar lavage fluid (BALF) and lung tissues. Histopathological studies were conducted to examine lung tissue-stained architecture. Our findings suggested that naringenin and morin significantly suppressed IL-5 and ROS production via various pathways. Interestingly, by reducing NFAT activity, naringenin and morin stimulated HO-1 expression, thereby suppressing IL-5 secretion due to regulating the transcription factor Nrf2 via P13/Akt or ERK/JNK signalling pathways in EL-4 cells, demonstrating the involvement of HO-1 expression in inhibiting asthmatic inflammation. The increased inflammatory cells in the BALF were substantially decreased by both naringenin and morin, followed by inhibition in the elevated Th-2 cytokines levels. The TNF-α protein levels in an allergic asthma mouse model were significantly reduced by suppressing Akt phosphorylation and eosinophil formation. Recent findings confirmed that naringenin and morin possess the potential to control asthma-related immune responses through antioxidant and anti-inflammatory properties, indicating potential therapeutic agents or functional foods.
Keyword
Figure
Reference
-
1. Chung MJ, Park JK, Park YI. 2012; Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int Immunopharmacol. 12:453–459. DOI: 10.1016/j.intimp.2011.12.027. PMID: 22266066.
Article2. Berend N, Salome CM, King GG. 2008; Mechanisms of airway hyperresponsiveness in asthma. Respirology. 13:624–631. DOI: 10.1111/j.1440-1843.2008.01330.x. PMID: 18713086.
Article3. Cockcroft DW, Davis BE. 2006; Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 118:551–559. quiz 560–561. DOI: 10.1016/j.jaci.2006.07.012. PMID: 16950269.
Article4. Gong JH, Shin D, Han SY, Kim JL, Kang YH. 2012; Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J Nutr. 142:47–56. DOI: 10.3945/jn.111.150748. PMID: 22157542.
Article5. Goh FY, Upton N, Guan S, Cheng C, Shanmugam MK, Sethi G, Leung BP, Wong WS. 2012; Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-κB. Eur J Pharmacol. 679:109–116. DOI: 10.1016/j.ejphar.2012.01.002. PMID: 22290391.
Article6. Hoefig KP, Heissmeyer V. 2018; Posttranscriptional regulation of T helper cell fate decisions. J Cell Biol. 217:2615–2631. DOI: 10.1083/jcb.201708075. PMID: 29685903. PMCID: PMC6080923.
Article7. Cousins DJ, McDonald J, Lee TH. 2008; Therapeutic approaches for control of transcription factors in allergic disease. J Allergy Clin Immunol. 121:803–809. quiz 810–811. DOI: 10.1016/j.jaci.2008.02.008. PMID: 18395546.
Article8. Kim JJ, Cho HW, Park HR, Jung U, Jo SK, Yee ST. 2013; Preventative effect of an herbal preparation (HemoHIM) on development of airway inflammation in mice via modulation of Th1/2 cells differentiation. PLoS One. 8:e68552. DOI: 10.1371/journal.pone.0068552. PMID: 23844220. PMCID: PMC3699527.
Article9. Rothenberg ME, Hogan SP. 2006; The eosinophil. Annu Rev Immunol. 24:147–174. DOI: 10.1146/annurev.immunol.24.021605.090720. PMID: 16551246.
Article10. Lambrecht BN, Hammad H. 2015; The immunology of asthma. Nat Immunol. 16:45–56. DOI: 10.1038/ni.3049. PMID: 25521684.
Article11. Iijima H, Duguet A, Eum SY, Hamid Q, Eidelman DH. 2001; Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am J Respir Crit Care Med. 163:1233–1240. DOI: 10.1164/ajrccm.163.5.2003145. PMID: 11316664.
Article12. Choi Y, Le Pham D, Lee DH, Lee SH, Kim SH, Park HS. 2018; Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma. Exp Mol Med. 50:1–8. DOI: 10.1038/s12276-018-0136-8. PMID: 30115903. PMCID: PMC6095846.
Article13. Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa'ad AH, Rothenberg ME. 2008; Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 121:1473–1483.e1-e4. DOI: 10.1016/j.jaci.2008.02.033. PMID: 18410960. PMCID: PMC2749495.
Article14. Foster PS, Hogan SP, Yang M, Mattes J, Young IG, Matthaei KI, Kumar RK, Mahalingam S, Webb DC. 2002; Interleukin-5 and eosinophils as therapeutic targets for asthma. Trends Mol Med. 8:162–167. DOI: 10.1016/S1471-4914(02)02302-X. PMID: 11927273.
Article15. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. 2009; Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 360:973–984. DOI: 10.1056/NEJMoa0808991. PMID: 19264686. PMCID: PMC3992367.
Article16. Messerli FH. 2012; Chocolate consumption, cognitive function, and Nobel laureates. N Engl J Med. 367:1562–1564. DOI: 10.1056/NEJMon1211064. PMID: 23050509.
Article17. Garcia V, Arts IC, Sterne JA, Thompson RL, Shaheen SO. 2005; Dietary intake of flavonoids and asthma in adults. Eur Respir J. 26:449–452. DOI: 10.1183/09031936.05.00142104. PMID: 16135726.
Article18. Shaheen SO, Sterne JA, Thompson RL, Songhurst CE, Margetts BM, Burney PG. 2001; Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med. 164(10 Pt 1):1823–1828. DOI: 10.1164/ajrccm.164.10.2104061. PMID: 11734430.19. Mishra V, Banga J, Silveyra P. 2018; Oxidative stress and cellular pathways of asthma and inflammation: therapeutic strategies and pharmacological targets. Pharmacol Ther. 181:169–182. DOI: 10.1016/j.pharmthera.2017.08.011. PMID: 28842273. PMCID: PMC5743757.
Article20. Higa S, Hirano T, Kotani M, Matsumoto M, Fujita A, Suemura M, Kawase I, Tanaka T. 2003; Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J Allergy Clin Immunol. 111:1299–1306. DOI: 10.1067/mai.2003.1456. PMID: 12789233.21. Chin LH, Hon CM, Chellappan DK, Chellian J, Madheswaran T, Zeeshan F, Awasthi R, Aljabali AA, Tambuwala MM, Dureja H, Negi P, Kapoor DN, Goyal R, Paudel KR, Satija S, Gupta G, Hsu A, Wark P, Mehta M, Wadhwa R, et al. 2020; Molecular mechanisms of action of naringenin in chronic airway diseases. Eur J Pharmacol. 879:173139. DOI: 10.1016/j.ejphar.2020.173139. PMID: 32343971.
Article22. Moon PD, Choi IH, Kim HM. 2011; Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. Eur J Pharmacol. 671:128–132. DOI: 10.1016/j.ejphar.2011.09.163. PMID: 21963452.
Article23. Franova S, Kazimierova I, Pappova L, Joskova M, Plank L, Sutovska M. 2016; Bronchodilatory, antitussive and anti-inflammatory effect of morin in the setting of experimentally induced allergic asthma. J Pharm Pharmacol. 68:1064–1072. DOI: 10.1111/jphp.12576. PMID: 27283356.
Article24. Iwamura C, Shinoda K, Yoshimura M, Watanabe Y, Obata A, Nakayama T. 2010; Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol Int. 59:67–73. DOI: 10.2332/allergolint.09-OA-0118. PMID: 20035147.
Article25. Seyedrezazadeh E, Kolahian S, Shahbazfar AA, Ansarin K, Pour Moghaddam M, Sakhinia M, Sakhinia E, Vafa M. 2015; Effects of the flavanone combination hesperetin-naringenin, and orange and grapefruit juices, on airway inflammation and remodeling in a murine asthma model. Phytother Res. 29:591–598. DOI: 10.1002/ptr.5292. PMID: 25640915.
Article26. Shi R, Xu JW, Xiao ZT, Chen RF, Zhang YL, Lin JB, Cheng KL, Wei GY, Li PB, Zhou WL, Su WW. 2019; Naringin and Naringenin relax rat tracheal smooth by regulating BKCa activation. J Med Food. 22:963–970. DOI: 10.1089/jmf.2018.4364. PMID: 31259654.27. Shi Y, Dai J, Liu H, Li RR, Sun PL, Du Q, Pang LL, Chen Z, Yin KS. 2009; Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can J Physiol Pharmacol. 87:729–735. DOI: 10.1139/Y09-065. PMID: 19794524.28. Shi Y, Tan Y, Mao S, Gu W. 2014; Naringenin inhibits allergen-induced airway remodeling in a murine model of asthma. Mol Med Rep. 9:1204–1208. DOI: 10.3892/mmr.2014.1940. PMID: 24534822.
Article29. Ma Y, Ge A, Zhu W, Liu YN, Ji NF, Zha WJ, Zhang JX, Zeng XN, Huang M. 2016; Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxid Med Cell Longev. 2016:5843672. DOI: 10.1155/2016/5843672. PMID: 26783416. PMCID: PMC4691473.
Article30. Chen YH, Chen HY, Hsu CL, Yen GC. 2007; Induction of apoptosis by the Lactuca indica L. in human leukemia cell line and its active components. J Agric Food Chem. 55:1743–1749. DOI: 10.1021/jf063118t. PMID: 17295517.31. Ho CY, Cheng YT, Chau CF, Yen GC. 2012; Effect of diallyl sulfide on in vitro and in vivo Nrf2-mediated pulmonic antioxidant enzyme expression via activation ERK/p38 signaling pathway. J Agric Food Chem. 60:100–107. DOI: 10.1021/jf203800d. PMID: 22118872.32. Chang HY, Chen SY, Wu CH, Lu CC, Yen GC. 2019; Glycyrrhizin attenuates the process of epithelial-to-mesenchymal transition by modulating HMGB1 initiated novel signaling pathway in prostate cancer cells. J Agric Food Chem. 67:3323–3332. DOI: 10.1021/acs.jafc.9b00251. PMID: 30832473.
Article33. Cheng YT, Lin JA, Jhang JJ, Yen GC. 2019; Protocatechuic acid-mediated DJ-1/PARK7 activation followed by PI3K/mTOR signaling pathway activation as a novel mechanism for protection against ketoprofen-induced oxidative damage in the gastrointestinal mucosa. Free Radic Biol Med. 130:35–47. DOI: 10.1016/j.freeradbiomed.2018.10.415. PMID: 30326282.
Article34. Altman LC, Ayars GH, Baker C, Luchtel DL. 1993; Cytokines and eosinophil-derived cationic proteins upregulate intercellular adhesion molecule-1 on human nasal epithelial cells. J Allergy Clin Immunol. 92:527–536. DOI: 10.1016/0091-6749(93)90077-S. PMID: 8104967.
Article35. Wambre E, Bajzik V, DeLong JH, O'Brien K, Nguyen QA, Speake C, Gersuk VH, DeBerg HA, Whalen E, Ni C, Farrington M, Jeong D, Robinson D, Linsley PS, Vickery BP, Kwok WW. 2017; A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 9:eaam9171. DOI: 10.1126/scitranslmed.aam9171. PMID: 28768806. PMCID: PMC5987220.
Article36. Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, Tumes DJ, Okamoto Y. 2017; TH2 cells in health and disease. Annu Rev Immunol. 35:53–84. DOI: 10.1146/annurev-immunol-051116-052350. PMID: 27912316.37. Cousins DJ. 2017; Pinning allergies on pathogenic TH2 cells. Sci Transl Med. 9:eaao0392. DOI: 10.1126/scitranslmed.aao0392. PMID: 28768801.38. Toscano-Garibay JD, Arriaga-Alba M, Sánchez-Navarrete J, Mendoza-García M, Flores-Estrada JJ, Moreno-Eutimio MA, Espinosa-Aguirre JJ, González-Ávila M, Ruiz-Pérez NJ. 2017; Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci Rep. 7:11479. DOI: 10.1038/s41598-017-11818-5. PMID: 28904369. PMCID: PMC5597616.
Article39. Frabasile S, Koishi AC, Kuczera D, Silveira GF, Verri WA Jr, Duarte Dos Santos CN, Bordignon J. 2017; Corrigendum: The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci Rep. 7:43976. DOI: 10.1038/srep43976. PMID: 28290539. PMCID: PMC5349511.
Article40. Mosqueda-Solís A, Sánchez J, Reynés B, Palou M, Portillo MP, Palou A, Picó C. 2018; Hesperidin and capsaicin, but not the combination, prevent hepatic steatosis and other metabolic syndrome-related alterations in Western diet-fed rats. Sci Rep. 8:15100. DOI: 10.1038/s41598-018-32875-4. PMID: 30305645. PMCID: PMC6180094.
Article41. Park JH, Ku HJ, Kim JK, Park JW, Lee JH. 2018; Amelioration of high fructose-induced cardiac hypertrophy by Naringin. Sci Rep. 8:9464. DOI: 10.1038/s41598-018-27788-1. PMID: 29930336. PMCID: PMC6013481.
Article42. Liu X, Wang N, Fan S, Zheng X, Yang Y, Zhu Y, Lu Y, Chen Q, Zhou H, Zheng J. 2016; The citrus flavonoid Naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci Rep. 6:39735. DOI: 10.1038/srep39735. PMID: 28004841. PMCID: PMC5177915.
Article43. Dusabimana T, Kim SR, Kim HJ, Park SW, Kim H. 2019; Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp Mol Med. 51:1–16. DOI: 10.1038/s12276-019-0245-z. PMID: 31028246. PMCID: PMC6486618.
Article44. Anwar A, Masri A, Rao K, Rajendran K, Khan NA, Shah MR, Siddiqui R. 2019; Antimicrobial activities of green synthesized gums-stabilized nanoparticles loaded with flavonoids. Sci Rep. 9:3122. DOI: 10.1038/s41598-019-39528-0. PMID: 30816269. PMCID: PMC6395601.
Article45. Fric J, Zelante T, Wong AY, Mertes A, Yu HB, Ricciardi-Castagnoli P. 2012; NFAT control of innate immunity. Blood. 120:1380–1389. DOI: 10.1182/blood-2012-02-404475. PMID: 22611159.
Article46. Crabtree GR, Olson EN. 2002; NFAT signaling: choreographing the social lives of cells. Cell. 109 Suppl:S67–S79. DOI: 10.1016/S0092-8674(02)00699-2. PMID: 11983154.47. Ogawa K, Kaminuma O, Okudaira H, Kikkawa H, Ikezawa K, Sakurai N, Mori A. 2002; Transcriptional regulation of the IL-5 gene in peripheral T cells of asthmatic patients. Clin Exp Immunol. 130:475–483. DOI: 10.1046/j.1365-2249.2002.01994.x. PMID: 12452838. PMCID: PMC1906553.
Article48. Wang C, Choi YH, Xian Z, Zheng M, Piao H, Yan G. 2018; Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int Immunopharmacol. 65:571–579. DOI: 10.1016/j.intimp.2018.11.003. PMID: 30415164.
Article49. Chung MJ, Sohng JK, Choi DJ, Park YI. 2013; Inhibitory effect of phloretin and biochanin A on IgE-mediated allergic responses in rat basophilic leukemia RBL-2H3 cells. Life Sci. 93:401–408. DOI: 10.1016/j.lfs.2013.07.019. PMID: 23907072.
Article50. Hazlewood LC, Wood LG, Hansbro PM, Foster PS. 2011; Dietary lycopene supplementation suppresses TH2 responses and lung eosinophilia in a mouse model of allergic asthma. J Nutr Biochem. 22:95–100. DOI: 10.1016/j.jnutbio.2009.12.003. PMID: 20392623.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancement of Allergen-induced Airway Inflammation by NOX2 Deficiency
- Effect of TiOâ‚‚ Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness
- Anti-Allergic Effect of Oroxylin A from Oroxylum indicum Using in vivo and in vitro Experiments
- Effect of DHEA Ingestion on Atopic Dermatitis-like Lesion in BALB/c Mice Sensitized by Ovalbumin
- Anti-inflammatory Effect of Alloferon on Ovalbumin-induced Asthma