Allergy Asthma Immunol Res.
2017 May;9(3):257-264. 10.4168/aair.2017.9.3.257.
Effect of TiOâ‚‚ Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness
- Affiliations
-
- 1Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. jas877@schmc.ac.kr
- 2Department of Otolayngology, Seoul National Uiversity, Seoul, Korea.
- KMID: 2371726
- DOI: http://doi.org/10.4168/aair.2017.9.3.257
Abstract
- PURPOSE
Nanoparticles (NPs) may cause cell and tissue damage, leading to local and systemic inflammatory responses and adverse effects on health due to the inhalation of particulate matter. The inflammasome is a major regulator of inflammation through its activation of pro-caspase-1, which cleaves pro-interleukin-1β (pro-IL-1β) into its mature form and may induce acute and chronic immune responses to NPs. However, little is known about the response of the inflammasome to NP exposure via the airways in asthma. The aim of this study was to identify the impact of titanium dioxide (TiO2) NPs on inflammasome in a mouse model of allergic asthma.
METHODS
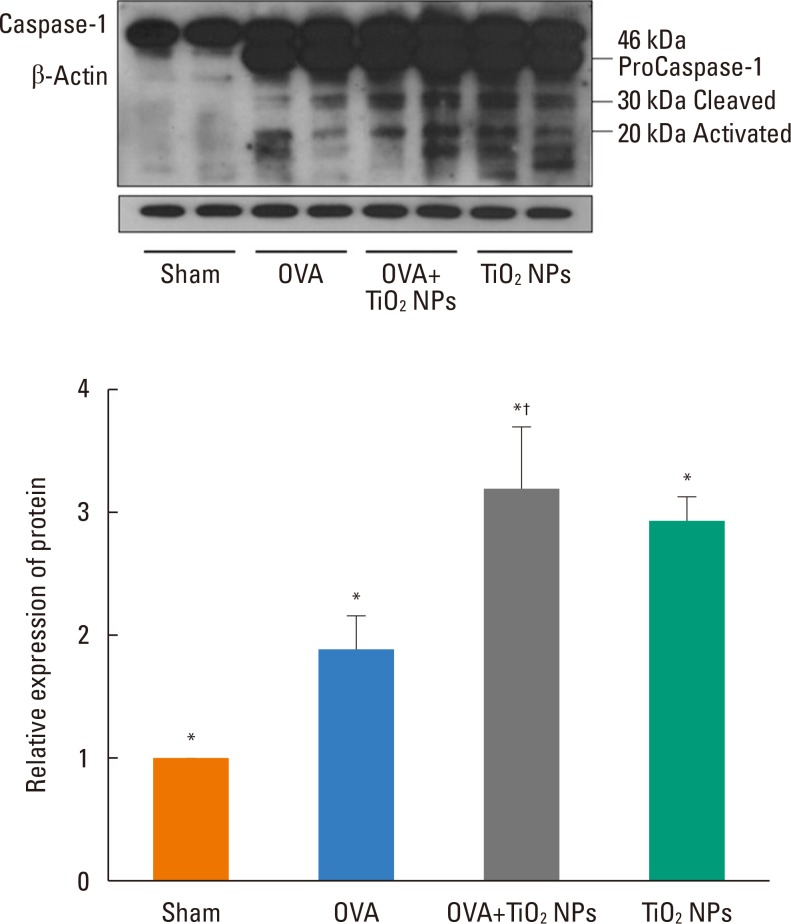
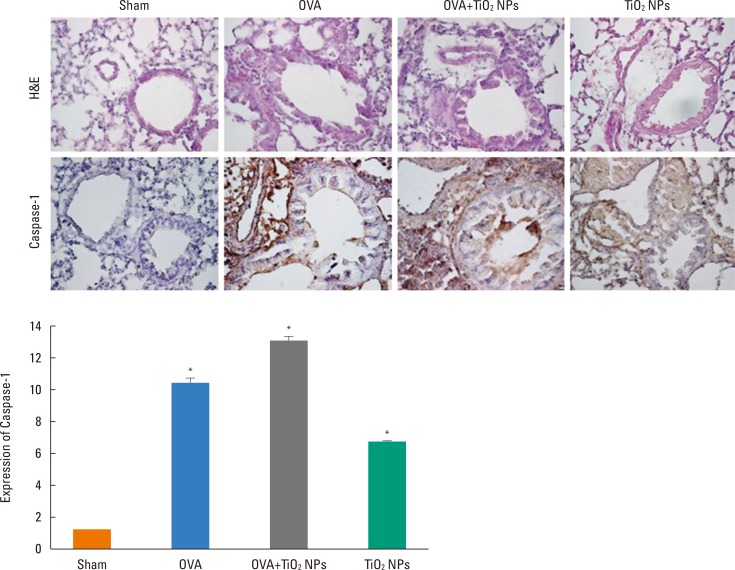
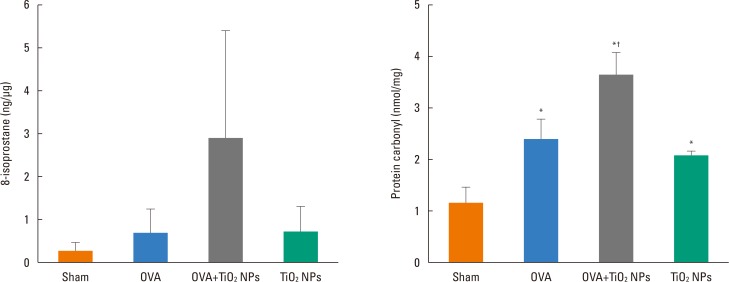
Mice were treated with ovalbumin (OVA) or TiOâ‚‚ NPs. IL-1β, IL-18, NAIP, CIITA, HET-E, TP-2 (NACHT), leucine-rich repeat (LRR), pyrin domain-containing protein 3 (NLRP3), and caspase-1 were assessed by Western blotting. Caspase-1 was assessed by immunohistochemistry (IHC). Levels of reactive oxygen species (ROS)"”as markers of oxidative damage"”and the mediators 8-isoprostane and carbonyl were measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS
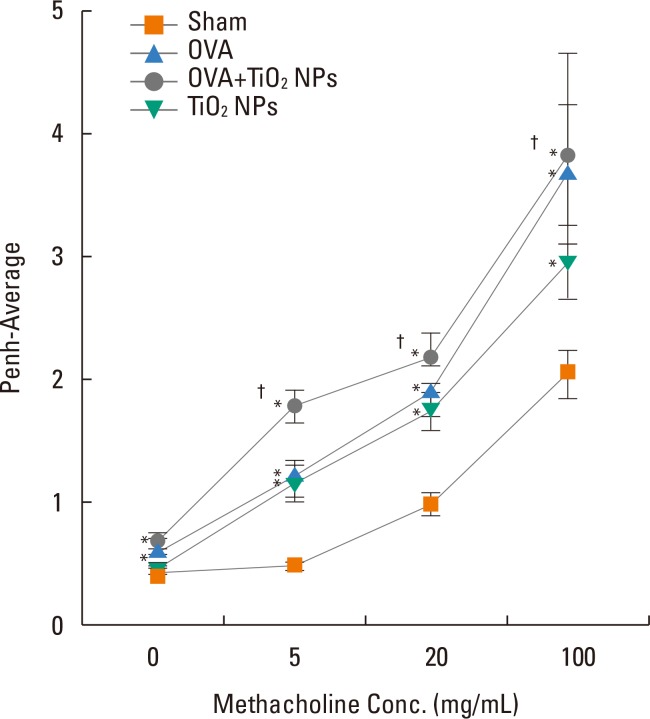
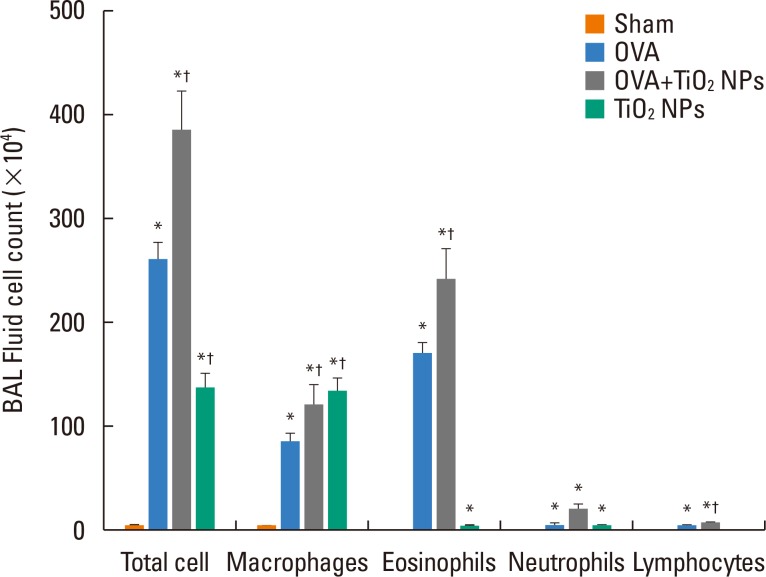
Airway hyperresponsiveness (AHR) and inflammation were increased in OVA-sensitized/challenged mice, and these responses were exacerbated by exposure to TiO₂ NPs. NP treatment increased IL-1β and IL-18 expression in OVA-sensitized/challenged mice. NPs augmented the expression of NLRP3 and caspase-1, leading to production of active caspase-1 in the lung. Caspase-1 expression was increased and exacerbated by TiO₂ NP exposure in OVA-sensitized/challenged mice. ROS levels tended to be increased in OVA-sensitized/challenged and OVA-sensitized/challenged-plus-TiO₂ NP-exposed mice.
CONCLUSIONS
Our data demonstrated that inflammasome activation occured in asthmatic lungs following NP exposure, suggesting that targeting the inflammasome may assist in controling NP-induced airway inflammation and hyperresponsiveness.
Keyword
MeSH Terms
-
Animals
Asthma
Blotting, Western
Caspase 1
Enzyme-Linked Immunosorbent Assay
Immunohistochemistry
Inflammasomes
Inflammation*
Inhalation
Interleukin-18
Lung
Mice
Nanoparticles*
Ovalbumin
Particulate Matter
Reactive Oxygen Species
Titanium
Caspase 1
Inflammasomes
Interleukin-18
Ovalbumin
Particulate Matter
Reactive Oxygen Species
Titanium
Figure
Reference
-
1. Brusselle GG, Provoost S, Bracke KR, Kuchmiy A, Lamkanfi M. Inflammasomes in respiratory disease: from bench to bedside. Chest. 2014; 145:1121–1133. PMID: 24798836.2. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012; 18:673–683. PMID: 22561831.
Article3. Müzes G, Sipos F. Inflammasome, inflammation and cancer: an interrelated pathobiological triad. Curr Drug Targets. 2015; 16:249–257. PMID: 25547909.4. dos Santos G, Kutuzov MA, Ridge KM. The inflammasome in lung diseases. Am J Physiol Lung Cell Mol Physiol. 2012; 303:L627–L633. PMID: 22904168.
Article5. Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015; 22:1111–1129. PMID: 25330206.
Article6. Bose S, Segovia JA, Somarajan SR, Chang TH, Kannan TR, Baseman JB. ADP-ribosylation of NLRP3 by mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. MBio. 2014; 23:e02186–e02114.
Article7. Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007; 2:MR17–MR71. PMID: 20419892.
Article9. See SW, Balasubramanian R. Risk assessment of exposure to indoor aerosols associated with Chinese cooking. Environ Res. 2006; 102:197–204. PMID: 16457802.
Article10. Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res. 2014; 129:11–19. PMID: 24528997.
Article11. Gong H Jr, Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, et al. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal Toxicol. 2008; 20:533–545. PMID: 18444007.
Article12. Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, et al. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol. 2010; 299:L374–L383. PMID: 20562226.
Article13. Moon KY, Park MK, Leikauf GD, Park CS, Jang AS. Diesel exhaust particle-induced airway responses are augmented in obese rats. Int J Toxicol. 2014; 33:21–28. PMID: 24536021.
Article14. Moon KY, Lee PH, Kim BG, Park CS, Leikauf GD, Jang AS. Claudin 5 in a murine model of allergic asthma: its implication and response to steroid treatment. J Allergy Clin Immunol. 2015; 136:1694–1696.e1-5. PMID: 26409663.
Article15. Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282:2261–2263. PMID: 9856950.
Article16. Cha MH, Rhim T, Kim KH, Jang AS, Paik YK, Park CS. Proteomic identification of macrophage migration-inhibitory factor upon exposure to TiO2 particles. Mol Cell Proteomics. 2007; 6:56–63. PMID: 17028300.
Article17. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997; 156:766–775. PMID: 9309991.
Article18. Huang YJ. The respiratory microbiome and innate immunity in asthma. Curr Opin Pulm Med. 2015; 21:27–32. PMID: 25405668.
Article19. Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014; 151:1–15. PMID: 24503171.
Article20. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015; 21:677–687. PMID: 26121197.
Article21. Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013; 187:1067–1075. PMID: 23491408.
Article22. Philip NH, Artis D. New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol Cell Biol. 2013; 91:225–231. PMID: 23337700.
Article23. Shin S, Brodsky IE. The inflammasome: learning from bacterial evasion strategies. Semin Immunol. 2015; 27:102–110. PMID: 25914126.
Article24. Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014; 43:1067–1076. PMID: 24136334.
Article25. Hirota JA, Hirota SA, Warner SM, Stefanowicz D, Shaheen F, Beck PL, et al. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. J Allergy Clin Immunol. 2012; 129:1116–1125.e6. PMID: 22227418.
Article26. Murphy A, Casey A, Byrne G, Chambers G, Howe O. Silver nanoparticles induce pro-inflammatory gene expression and inflammasome activation in human monocytes. J Appl Toxicol. 2016; 36:1311–1320. PMID: 26968431.
Article27. Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015; 6:e1629. PMID: 25654762.
Article28. Sandberg WJ, Låg M, Holme JA, Friede B, Gualtieri M, Kruszewski M, et al. Comparison of non-crystalline silica nanoparticles in IL-1β release from macrophages. Part Fibre Toxicol. 2012; 9:32. PMID: 22882971.
Article29. Mankan AK, Dau T, Jenne D, Hornung V. The NLRP3/ASC/Caspase-1 axis regulates IL-1β processing in neutrophils. Eur J Immunol. 2012; 42:710–715. PMID: 22213227.
Article30. Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010; 30:628–631. PMID: 20589420.
Article31. Kumar S, Verma MK, Srivastava AK. Ultrafine particles in urban ambient air and their health perspectives. Rev Environ Health. 2013; 28:117–128. PMID: 24192498.
Article32. Westerdahl D, Fruin S, Sax T, Fine PM, Sioutas C. Mobile platform measurements of ultrafine particles and associated pollutant concentrations on freeways and residential streets in Los Angeles. Atmos Environ (1994). 2005; 39:3597–3610.
Article33. Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ Health Perspect. 2005; 113:947–955. PMID: 16079062.
Article34. Evelyn A, Mannick S, Sermon PA. Unusual carbon-based nanofibers and chains among diesel-emitted particles. Nano Lett. 2003; 3:63–64.
Article35. Soto KF, Carrasco A, Powell TG, Garza KM, Murr LE. Comparative in vitro cytotoxicity assessment of some manufacturednanoparticulate materials characterized by transmissionelectron microscopy. J Nanopart Res. 2005; 7:145–169.
Article36. Kim BG, Lee PH, Lee SH, Kim YE, Shin MY, Kang Y, et al. Long-term effects of diesel exhaust particles on airway inflammation and remodeling in a mouse model. Allergy Asthma Immunol Res. 2016; 8:246–256. PMID: 26922935.
Article37. Seo S, Kim D, Min S, Paul C, Yoo Y, Choung JT. GIS-based association between PM10 and allergic diseases in Seoul: implications for health and environmental policy. Allergy Asthma Immunol Res. 2016; 8:32–40. PMID: 26540499.
Article38. Afshari A, Matson U, Ekberg LE. Characterization of indoor sources of fine and ultrafine particles: a study conducted in a full-scale chamber. Indoor Air. 2005; 15:141–150. PMID: 15737157.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Accelerated ecotoxicity of photoreactive nanoparticles on Moina macrocopa
- Effect of Particulate Matter on the NLRP3 Inflammasomes in Ocular Tissues and Cervical Lymph Nodes
- Biofilm formation on denture base resin including ZnO, CaO, and TiOâ‚‚ nanoparticles
- Preparation and characterization of rutile phase TiOâ‚‚ nanoparticles and their cytocompatibility with oral cancer cells
- Role of inflammasome activation in development and exacerbation of asthma