J Stroke.
2021 Sep;23(3):377-387. 10.5853/jos.2021.00619.
Impact of Multiphase Computed Tomography Angiography for Endovascular Treatment Decision-Making on Outcomes in Patients with Acute Ischemic Stroke
- Affiliations
-
- 1Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
- 2Department of Radiology, University Hospital of Basel, Basel, Switzerland
- 3Czech National Centre for Evidence-Based Healthcare and Knowledge Translation (Cochrane Czech Republic, Czech EBHC: JBI Centre of Excellence, Masaryk University GRADE Centre), Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Brno, Czech Republic
- 4Department of Neurology, University Hospital Ostrava, Ostrava-Poruba, Czech Republic
- 5Department of Experimental and Clinical Biomedical Sciences, University of Florence, Florence, Italy
- 6Department of Neurology, Hospital Vall d´Hebron, Barcelona, Spain
- 7Department of Clinical Neurosciences, London Health Sciences Centre, University of Western Ontario, London, ON, Canada
- 8Department of Radiology, University of Manitoba, Winnipeg, MB, Canada
- 9Department of Radiology, University of Calgary, Calgary, AB, Canada
- 10Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
- 11Department of Community Health Sciences, University of Calgary, Calgary, AB, Canada
- 12Department of Medicine, University of Calgary, Calgary, AB, Canada
- KMID: 2520915
- DOI: http://doi.org/10.5853/jos.2021.00619
Abstract
- Background and Purpose
Various imaging paradigms are used for endovascular treatment (EVT) decision-making and outcome estimation in acute ischemic stroke (AIS). We aim to compare how these imaging paradigms perform for EVT patient selection and outcome estimation.
Methods
Prospective multi-center cohort study of patients with AIS symptoms with multi-phase computed tomography angiography (mCTA) and computed tomography perfusion (CTP) baseline imaging. mCTA-based EVT-eligibility was defined as presence of large vessel occlusion (LVO) and moderate-to-good collaterals on mCTA. CTP-based eligibility was defined as presence of LVO, ischemic core (defined on relative cerebral blood flow, absolute cerebral blood flow, and cerebral blood volume maps) <70 mL, mismatch-ratio >1.8, absolute mismatch >15 mL. EVT-eligibility and adjusted rates of good outcome (modified Rankin Scale 0–2) based on these imaging paradigms were compared.
Results
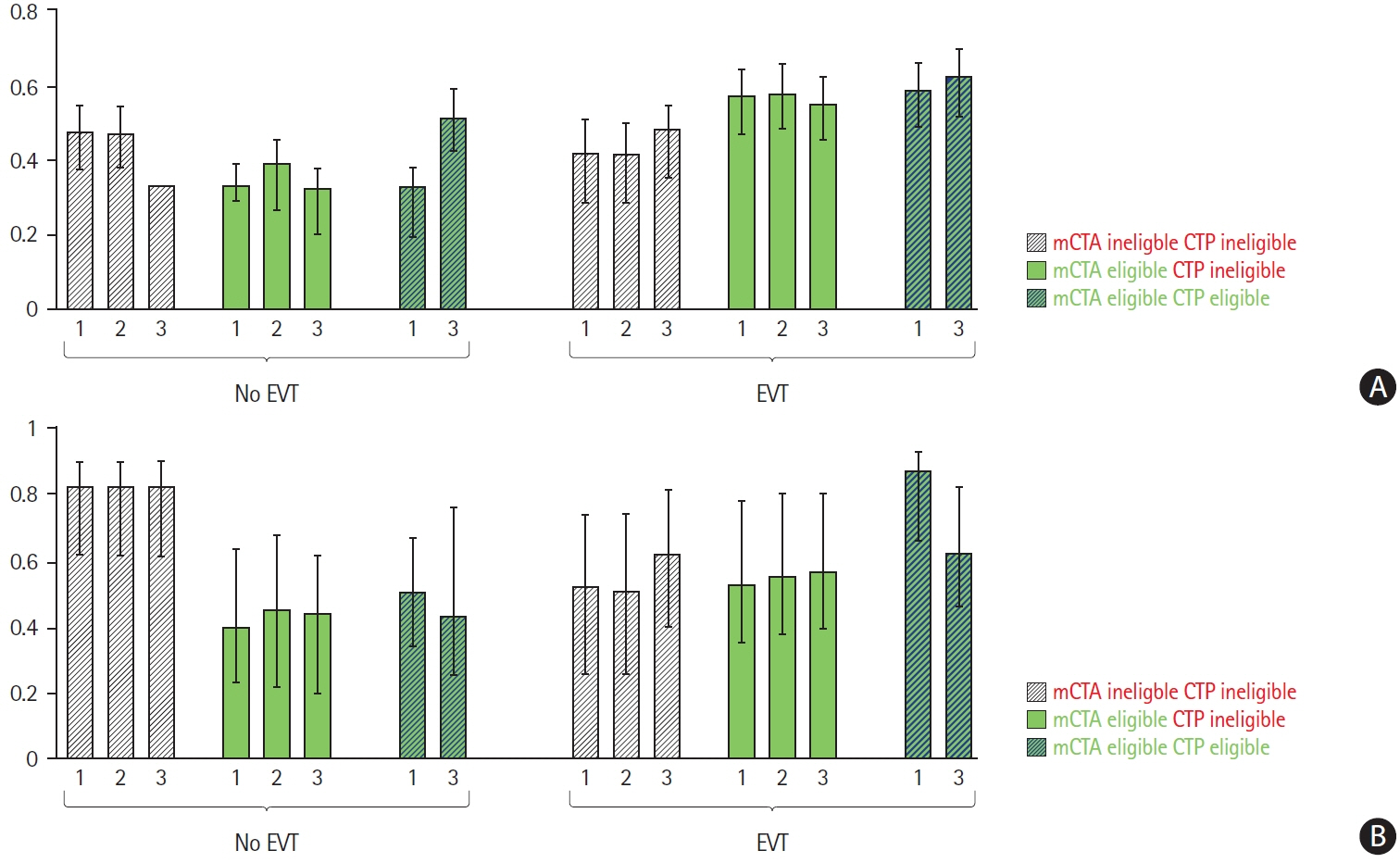
Of 289/464 patients with LVO, 263 (91%) were EVT-eligible by mCTA-criteria versus 63 (22%), 19 (7%) and 103 (36%) by rCBF, aCBF, and CBV-CTP-criteria. CTP and mCTA-criteria were discordant in 40% to 53%. Estimated outcomes were best in patients who met both mCTA and CTP eligibility-criteria and were treated with EVT (62% to 87% good outcome). Patients eligible for EVT by mCTA-criteria and not by CTP-criteria receiving EVT achieved good outcome rates of 53% to 57%. Few patients met CTP-criteria and not mCTA-criteria for EVT.
Conclusions
Simpler imaging selection criteria that rely on little else than detection of the occluded blood vessel may be more sensitive and less specific, thus resulting in more patients being offered EVT and arguably benefiting from it.
Figure
Reference
-
References
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20.2. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.3. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 372:1009–1018.
Article4. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372:2285–2295.
Article5. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21.6. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, OrtegaGutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.
Article7. Wang Z, Xie J, Tang TY, Zeng CH, Zhang Y, Zhao Z, et al. Collateral status at single-phase and multiphase CT angiography versus CT perfusion for outcome prediction in anterior circulation acute ischemic stroke. Radiology. 2020; 296:393–400.8. Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015; 275:510–520.
Article9. Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006; 37:979–985.10. Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006; 37:1334–1339.
Article11. d’Esterre CD, Boesen ME, Ahn SH, Pordeli P, Najm M, Minhas P, et al. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke. 2015; 46:3390–3397.
Article12. Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020; 395:878–887.13. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50:e344–e418.
Article14. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO): European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019; 11:535–538.
Article15. Olivot JM, Albucher JF, Guenego A, Thalamas C, Mlynash M, Rousseau V, et al. Mismatch profile influences outcome after mechanical thrombectomy. Stroke. 2021; 52:232–240.
Article16. Sarraj A, Hassan AE, Savitz S, Sitton C, Grotta J, Chen P, et al. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient’s selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol. 2019; 76:1147–1156.17. Jadhav AP, Hacke W, Dippel DWJ, Simonsen CZ, Costalat V, Fiehler J, et al. Select wisely: the ethical challenge of defining large core with perfusion in the early time window. J Neurointerv Surg. 2021; 13:497–499.
Article18. Kim B, Jung C, Nam HS, Kim BM, Kim YD, Heo JH, et al. Comparison between perfusion- and collateral-based triage for endovascular thrombectomy in a late time window. Stroke. 2019; 50:3465–3470.
Article19. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after largevessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.
Article20. Lopez-Rivera V, Abdelkhaleq R, Yamal JM, Singh N, Savitz SI, Czap AL, et al. Impact of initial imaging protocol on likelihood of endovascular stroke therapy. Stroke. 2020; 51:3055–3063.
Article21. Menon BK, Hill MD, Davalos A, Roos YB, Campbell BC, Dippel DW, et al. Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: metaanalysis of data from the HERMES Collaboration. J Neurointerv Surg. 2019; 11:1065–1069.
Article22. Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CB, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018; 17:895–904.23. Rodger M, Ramsay T, Fergusson D. Diagnostic randomized controlled trials: the final frontier. Trials. 2012; 13:137.
Article24. Grunwald IQ, Kulikovski J, Reith W, Gerry S, Namias R, Politi M, et al. Collateral automation for triage in stroke: evaluating automated scoring of collaterals in acute stroke on computed tomography scans. Cerebrovasc Dis. 2019; 47:217–222.
Article25. Almekhlafi MA, Kunz WG, McTaggart RA, Jayaraman MV, Najm M, Ahn SH, et al. Imaging triage of patients with latewindow (6-24 hours) acute ischemic stroke: a comparative study using multiphase CT angiography versus CT perfusion. Am J Neuroradiol. 2020; 41:129–133.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravenous Thrombolysis and Endovascular Thrombectomy in Acute Ischemic Stroke with Minor Symptom
- Dichotomizing Level of Pial Collaterals on Multiphase CT Angiography for Endovascular Treatment in Acute Ischemic Stroke: Should It Be Refined for 6-Hour Time Window?
- Choosing a Hyperacute Stroke Imaging Protocol for Proper Patient Selection and Time Efficient Endovascular Treatment: Lessons from Recent Trials
- Endovascular Treatment of Acute Ischemic Stroke
- Endovascular recanalization therapy for patients with acute ischemic stroke with hidden aortic dissection: A case series