Int J Stem Cells.
2021 Aug;14(3):262-274. 10.15283/ijsc20002.
Umbilical Cord Mesenchymal Stem Cells for Inhibiting the Fibrosis and Autoimmune Development in HOCl-Induced Systemic Scleroderma Mouse Model
- Affiliations
-
- 1Institute of Reproductive and Stem Cell Engineering, School of Basic Medical Sciences, Central South University, Changsha, China
- 2National Engineering Research Center of Human Stem Cells, Changsha, China
- 3Centre for Cardiovascular Sciences, Queen’s Medical Research Institute, School of Clinical Sciences, University of Edinburgh, Edinburgh, Scotland, UK
- KMID: 2519369
- DOI: http://doi.org/10.15283/ijsc20002
Abstract
- Background and Objectives
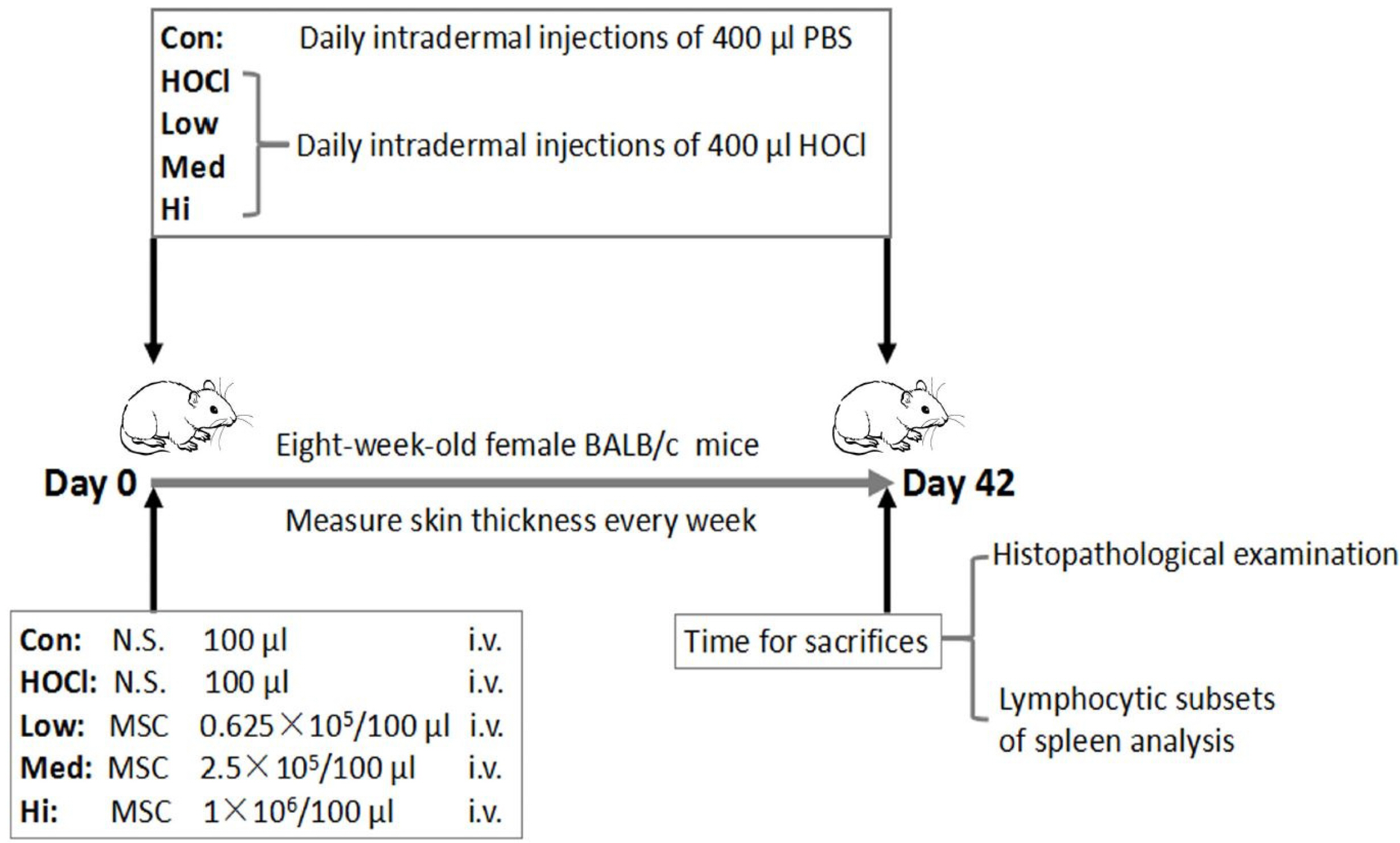
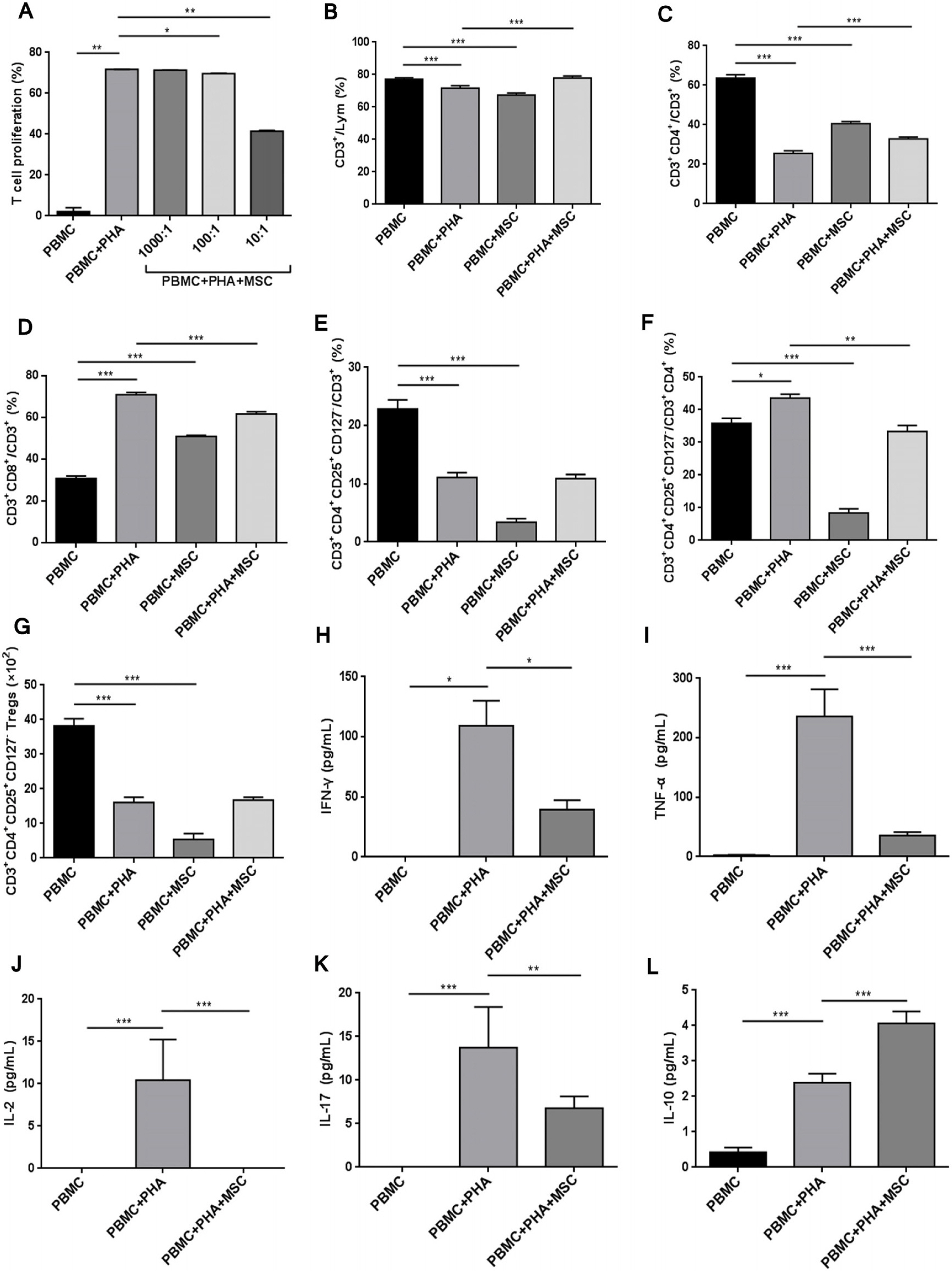
Systemic scleroderma (SSc) is a rare and serious connective tissue disease, an autoimmune disease, and a rare refractory disease. In this study, preventive effect of single systemic human umbilical cord mesenchymal stem cells (UC-MSCs) transfusion on SSc was preliminarily explored.
Methods and Results
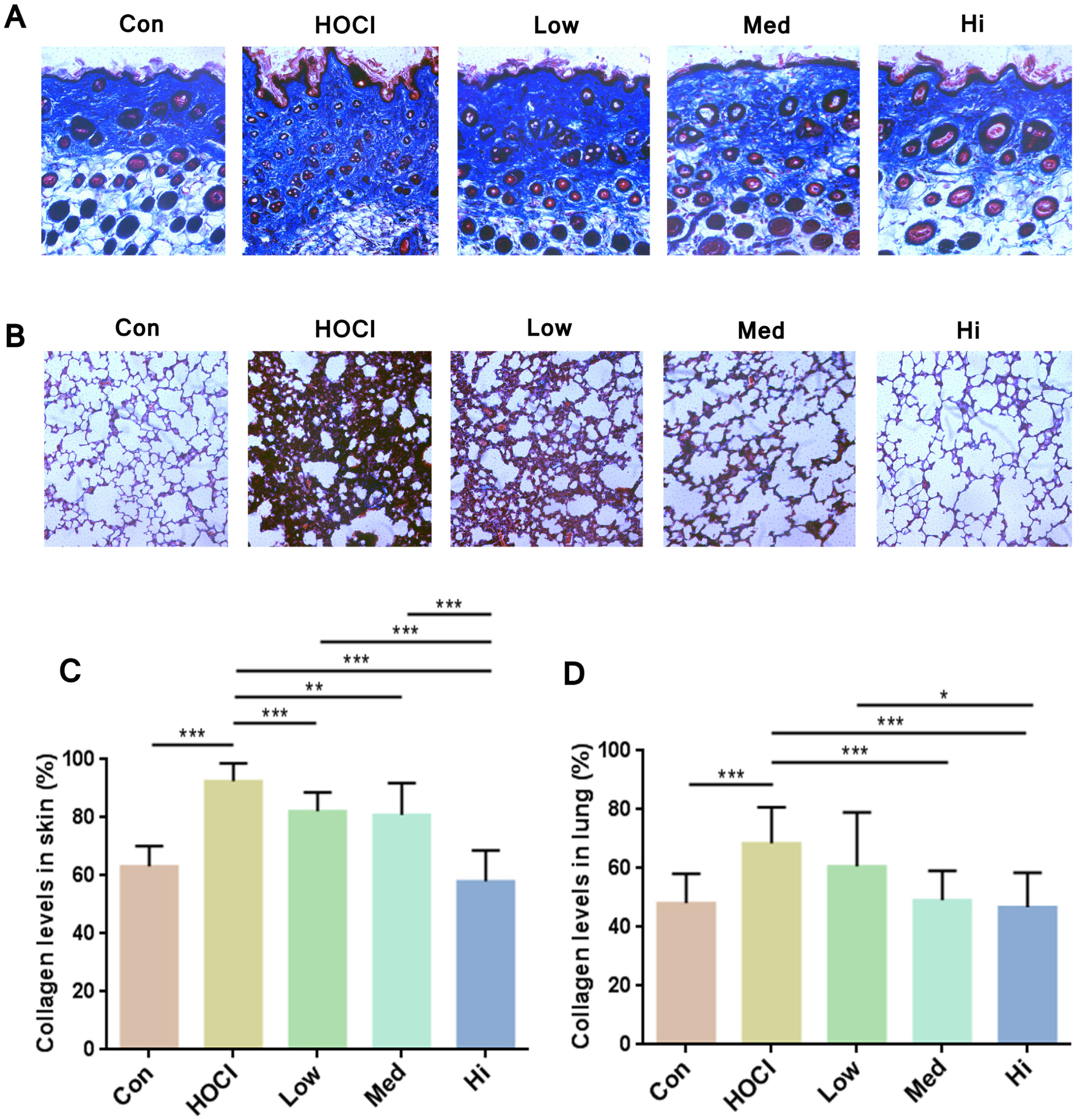
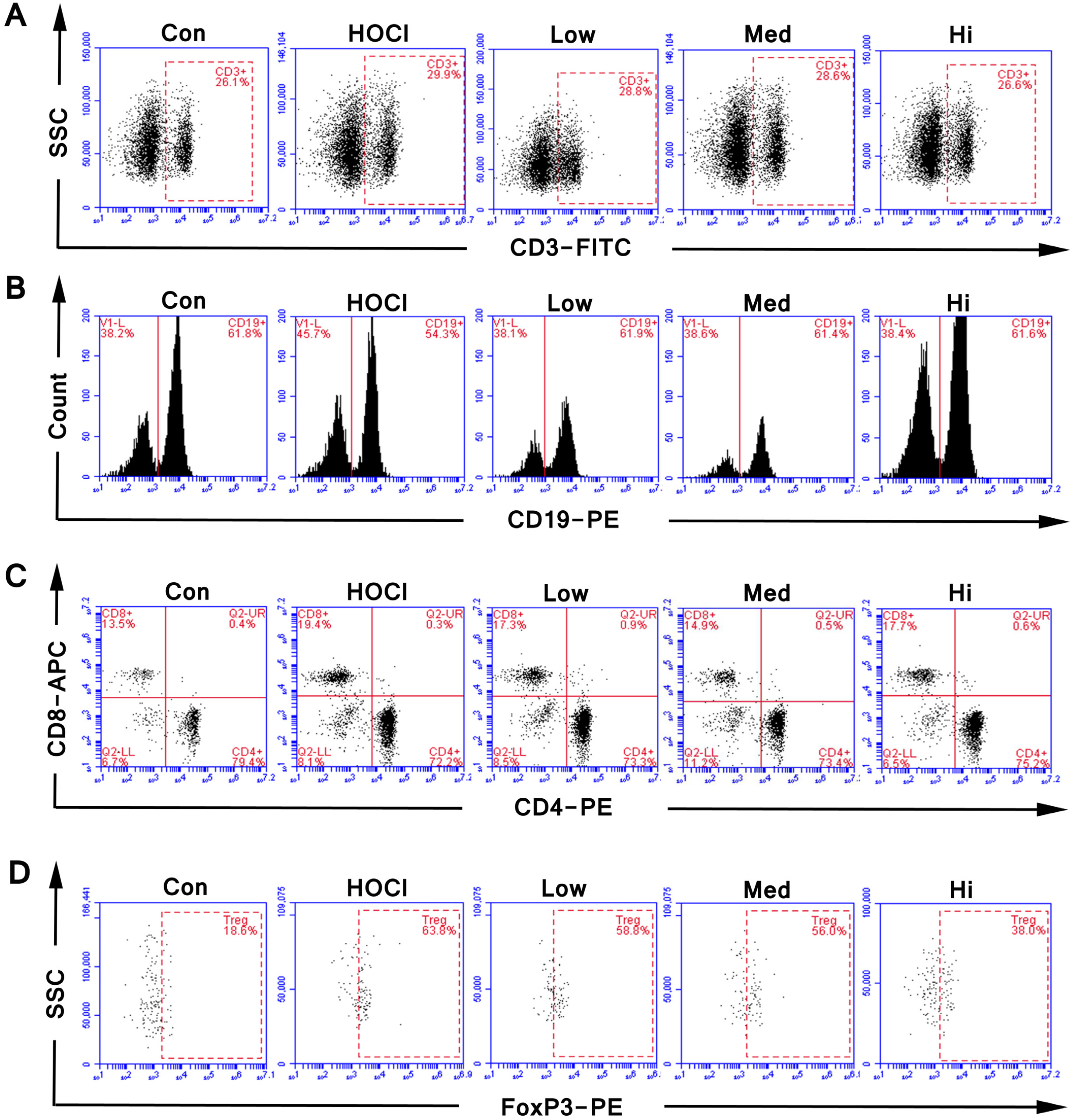
SSc mouse model was established by daily intradermal injection of Hypochlorite (HOCl). SSc mice were treated by single transfusion of UC-MSCs at 0.625×10 5 , 2.5×105 and 1×106 respectively. At the 42nd day of intradermal injection of HOCl, the symptoms showed up by skin and alveolar wall thickening, lymphocytic infiltration, increased collagen in skin/lung, and the increased proportion of CD3 + CD4+ CD25+ FoxP3+ cells (a Treg subset) in spleen. After UC-MSCs transfusion, the degree of skin thickening, alveolar wall thickening and lymphocyte infiltration were decreased, the collagen sedimentation in skin/lung was decreased, and the proportion of CD3+ CD4+ CD25+ FoxP3+ cells was decreased.
Conclusions
UC-MSC can achieve a preventive effect in SSc mice by fibrosis attenuation and immunoregulation.
Figure
Reference
-
References
1. Wu C, Chen Z, Dardalhon V, Xiao S, Thalhamer T, Liao M, Madi A, Franca RF, Han T, Oukka M, Kuchroo V. 2017; The transcription factor musculin promotes the unidirectional development of peripheral Treg cells by suppressing the TH2 transcriptional program. Nat Immunol. 18:344–353. DOI: 10.1038/ni.3667. PMID: 28114290. PMCID: PMC5591438.
Article2. Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. 2018; Mesenchymal stem cell exosomes promote immunosupp-ression of regulatory T cells in asthma. Exp Cell Res. 363:114–120. DOI: 10.1016/j.yexcr.2017.12.021. PMID: 29277503.
Article3. Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S, Cong Y, Chen H, Lu L, Shi S, Wang D, Chen W, Sun L. 2019; Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine. 45:341–350. DOI: 10.1016/j.ebiom.2019.06.016. PMID: 31248835. PMCID: PMC6642220.
Article4. Kim JY, Park M, Kim YH, Ryu KH, Lee KH, Cho KA, Woo SY. 2018; Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. J Tissue Eng Regen Med. 12:e1022–e1033. DOI: 10.1002/term.2423. PMCID: PMC6508967. PMID: 28107610.
Article5. Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM, Dijkstra G, van der Woude CJ, Roelen DL, Zwaginga JJ, Verspaget HW, Fibbe WE, Hommes DW, Peeters KCMJ, van der Meulen-de Jong AE. 2020; Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn's disease perianal fistulas. J Crohns Colitis. 14:64–70. DOI: 10.1093/ecco-jcc/jjz116. PMID: 31197361. PMCID: PMC6930001.
Article6. Martínez-Carrasco R, Sánchez-Abarca LI, Nieto-Gómez C, Martín García E, Sánchez-Guijo F, Argüeso P, Aijón J, Hernández-Galilea E, Velasco A. 2019; Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocul Surf. 17:285–294. DOI: 10.1016/j.jtos.2019.01.001. PMID: 30630121.
Article7. Rozier P, Maria A, Goulabchand R, Jorgensen C, Guilpain P, Noël D. 2018; Mesenchymal stem cells in systemic sclerosis: allogenic or autologous approaches for therapeutic use? Front Immunol. 9:2938. DOI: 10.3389/fimmu.2018.02938. PMID: 30619298. PMCID: PMC6302042.
Article8. van den Hombergh WM, Carreira PE, Knaapen-Hans HK, van den Hoogen FH, Fransen J, Vonk MC. 2016; An easy prediction rule for diffuse cutaneous systemic sclerosis using only the timing and type of first symptoms and auto-antibodies: derivation and validation. Rheumatology (Oxford). 55:2023–2032. DOI: 10.1093/rheumatology/kew305. PMID: 27550299.
Article9. Christopeit M, Schendel M, Föll J, Müller LP, Keysser G, Behre G. 2008; Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia. 22:1062–1064. DOI: 10.1038/sj.leu.2404996. PMID: 17972956.
Article10. Keyszer G, Christopeit M, Fick S, Schendel M, Taute BM, Behre G, Müller LP, Schmoll HJ. 2011; Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: report of five cases. Arthritis Rheum. 63:2540–2542. DOI: 10.1002/art.30431. PMID: 21547891.
Article11. Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. 2012; Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 10:544–555. DOI: 10.1016/j.stem.2012.03.007. PMID: 22542159. PMCID: PMC3348385.
Article12. Granel B, Daumas A, Jouve E, Harlé JR, Nguyen PS, Chabannon C, Colavolpe N, Reynier JC, Truillet R, Mallet S, Baiada A, Casanova D, Giraudo L, Arnaud L, Veran J, Sabatier F, Magalon G. 2015; Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis. 74:2175–2182. DOI: 10.1136/annrheumdis-2014-205681. PMID: 25114060. PMCID: PMC4680117.
Article13. Van Pham P, Truong NC, Le PT, Tran TD, Vu NB, Bui KH, Phan NK. 2016; Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical appli-cations. Cell Tissue Bank. 17:289–302. DOI: 10.1007/s10561-015-9541-6. PMID: 26679929.
Article14. Maria ATJ, Rozier P, Fonteneau G, Sutra T, Maumus M, Toupet K, Cristol JP, Jorgensen C, Guilpain P, Noël D. 2018; iNOS activity is required for the therapeutic effect of mesenchymal stem cells in experimental systemic sclerosis. Front Immunol. 9:3056. DOI: 10.3389/fimmu.2018.03056. PMID: 30622540. PMCID: PMC6308989.
Article15. Hughes M, Ong VH, Anderson ME, Hall F, Moinzadeh P, Griffiths B, Baildam E, Denton CP, Herrick AL. 2015; Consensus best practice pathway of the UK Scleroderma Study Group: digital vasculopathy in systemic sclerosis. Rheumatology (Oxford). 54:2015–2024. DOI: 10.1093/rheumatology/kev201. PMID: 26116156.
Article16. Cavagna L, Codullo V, Ghio S, Scirè CA, Guzzafame E, Scelsi L, Rossi S, Montecucco C, Caporali R. 2016; Undiagnosed connective tissue diseases: high prevalence in pulmonary arterial hypertension patients. Medicine (Baltimore). 95:e4827. DOI: 10.1097/MD.0000000000004827. PMID: 27684814. PMCID: PMC5265907.17. Giacomelli R, Liakouli V, Berardicurti O, Ruscitti P, Di Benedetto P, Carubbi F, Guggino G, Di Bartolomeo S, Ciccia F, Triolo G, Cipriani P. 2017; nterstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int. 37:853–863. DOI: 10.1007/s00296-016-3636-7. PMID: 28063071.
Article18. Capelli C, Zaccara E, Cipriani P, Di Benedetto P, Maglione W, Andracco R, Di Luca G, Pignataro F, Giacomelli R, Introna M, Vitali C, Del Papa N. 2017; Phenotypical and functional characteristics of in vitro-expanded adipose-derived mesenchymal stromal cells from patients with systematic sclerosis. Cell Transplant. 26:841–854. DOI: 10.3727/096368917X694822. PMID: 28139194. PMCID: PMC5657721.
Article19. Griffin M, Ryan CM, Pathan O, Abraham D, Denton CP, Butler PE. 2017; Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: implications for regenerative medicine. Stem Cell Res Ther. 8:23. DOI: 10.1186/s13287-016-0444-7. PMID: 28173869. PMCID: PMC5297142.
Article20. Soldano S, Trombetta AC, Contini P, Tomatis V, Ruaro B, Brizzolara R, Montagna P, Sulli A, Paolino S, Pizzorni C, Smith V, Cutolo M. 2018; Increase in circulating cells coexpressing M1 and M2 macrophage surface markers in patients with systemic sclerosis. Ann Rheum Dis. 77:1842–1845. DOI: 10.1136/annrheumdis-2018-213648. PMID: 30012643.
Article21. Taniguchi T, Asano Y, Akamata K, Noda S, Takahashi T, Ichimura Y, Toyama T, Trojanowska M, Sato S. 2015; Fibrosis, vascular activation, and immune abnormalities resembling systemic sclerosis in bleomycin-treated Fli-1-haploinsuffi-cient mice. Arthritis Rheumatol. 67:517–526. DOI: 10.1002/art.38948. PMID: 25385187. PMCID: PMC4342755.
Article22. Wuttge DM, Andreasson A, Tufvesson E, Johansson ÅC, Scheja A, Hellmark T, Hesselstrand R, Truedsson L. 2016; CD81 and CD48 show different expression on blood eosinophils in systemic sclerosis: new markers for disease and pulmonary inflammation? Scand J Rheumatol. 45:107–113. DOI: 10.3109/03009742.2015.1054877. PMID: 26926492.
Article23. Manetti M, Romano E, Rosa I, Guiducci S, Bellando-Randone S, De Paulis A, Ibba-Manneschi L, Matucci-Cerinic M. 2017; Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis. 76:924–934. DOI: 10.1136/annrheumdis-2016-210229. PMID: 28062404.
Article24. Koenig M, Bentow C, Satoh M, Fritzler MJ, Senécal JL, Mahler M. 2019; Autoantibodies to a novel Rpp38 (Th/To) derived B-cell epitope are specific for systemic sclerosis and associate with a distinct clinical phenotype. Rheumatology (Oxford). 58:1784–1793. DOI: 10.1093/rheumatology/kez123. PMID: 31323671.
Article25. Shu J, Pan L, Huang X, Wang P, Li H, He X, Cai Z. 2015; Transplantation of human amnion mesenchymal cells attenuates the disease development in rats with collagen-induced arthritis. Clin Exp Rheumatol. 33:484–490. PMID: 25962385.26. Thiel A, Yavanian G, Nastke MD, Morales P, Kouris NA, Kimbrel EA, Lanza R. 2015; Human embryonic stem cell-derived mesenchymal cells preserve kidney function and extend lifespan in NZB/W F1 mouse model of lupus nephritis. Sci Rep. 5:17685. DOI: 10.1038/srep17685. PMID: 26628350. PMCID: PMC4667213.
Article27. Li H, Guo ZK, Li XS, Hou CM, Tang PH, Mao N. 2007; Functional and phenotypic alteration of intrasplenic lymphocytes affected by mesenchymal stem cells in a murine allosplenocyte transfusion model. Cell Transplant. 16:85–95. DOI: 10.3727/000000007783464470. PMID: 17436858.
Article28. Ugor E, Simon D, Almanzar G, Pap R, Najbauer J, Németh P, Balogh P, Prelog M, Czirják L, Berki T. 2017; Increased proportions of functionally impaired regulatory T cell subsets in systemic sclerosis. Clin Immunol. 184:54–62. DOI: 10.1016/j.clim.2017.05.013. PMID: 28522286.
Article29. Negrini S, Fenoglio D, Parodi A, Kalli F, Battaglia F, Nasi G, Curto M, Tardito S, Ferrera F, Filaci G. 2017; Phenotypic alterations involved in CD8+ Treg impairment in systemic sclerosis. Front Immunol. 8:18. DOI: 10.3389/fimmu.2017.00018. PMID: 28154567. PMCID: PMC5243838.
Article30. Rosenbaum M, Gewies A, Pechloff K, Heuser C, Engleitner T, Gehring T, Hartjes L, Krebs S, Krappmann D, Kriegsmann M, Weichert W, Rad R, Kurts C, Ruland J. 2019; Bcl10-controlled Malt1 paracaspase activity is key for the immune suppressive function of regulatory T cells. Nat Commun. 10:2352. DOI: 10.1038/s41467-019-10203-2. PMID: 31138793. PMCID: PMC6538646.
Article31. Pathak S, Regmi S, Shrestha P, Choi I, Doh KO, Jeong JH. 2019; Mesenchymal stem cell capping on ECM-anchored caspase inhibitor-loaded PLGA microspheres for intraperitoneal injection in DSS-induced murine colitis. Small. 15:e1901269. DOI: 10.1002/smll.201901269. PMID: 31018047.
Article32. Radstake TR, van Bon L, Broen J, Wenink M, Santegoets K, Deng Y, Hussaini A, Simms R, Cruikshank WW, Lafyatis R. 2009; Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS One. 4:e5981. DOI: 10.1371/journal.pone.0005981. PMID: 19543397. PMCID: PMC2695559.33. Slobodin G, Ahmad MS, Rosner I, Peri R, Rozenbaum M, Kessel A, Toubi E, Odeh M. 2010; Regulatory T cells (CD4+CD25brightFoxP3+) expansion in systemic sclerosis correlates with disease activity and severity. Cell Immunol. 261:77–80. DOI: 10.1016/j.cellimm.2009.12.009. PMID: 20096404.
Article34. Groh ME, Maitra B, Szekely E, Koç ON. 2005; Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 33:928–934. DOI: 10.1016/j.exphem.2005.05.002. PMID: 16038786.
Article35. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. 2005; Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 105:2821–2827. DOI: 10.1182/blood-2004-09-3696. PMID: 15591115.
Article36. Aggarwal S, Pittenger MF. 2005; Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105:1815–1822. DOI: 10.1182/blood-2004-04-1559. PMID: 15494428.
Article37. Maria AT, Maumus M, Le Quellec A, Jorgensen C, Noël D, Guilpain P. 2017; Adipose-derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin Rev Allergy Immunol. 52:234–259. DOI: 10.1007/s12016-016-8552-9. PMID: 27207172.
Article38. Cras A, Farge D, Carmoi T, Lataillade JJ, Wang DD, Sun L. 2015; Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res Ther. 17:301. DOI: 10.1186/s13075-015-0819-7. PMID: 26525582. PMCID: PMC4631077.
Article39. Cutolo M, Ruaro B, Montagna P, Brizzolara R, Stratta E, Trombetta AC, Scabini S, Tavilla PP, Parodi A, Corallo C, Giordano N, Paolino S, Pizzorni C, Sulli A, Smith V, Soldano S. 2018; Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts. Arthritis Res Ther. 20:77. DOI: 10.1186/s13075-018-1577-0. PMID: 29720235. PMCID: PMC5932791.
Article40. Tan K, Zheng K, Li D, Lu H, Wang S, Sun X. 2017; Impact of adipose tissue or umbilical cord derived mesenchymal stem cells on the immunogenicity of human cord blood derived endothelial progenitor cells. PLoS One. 12:e0178624. DOI: 10.1371/journal.pone.0178624. PMID: 28562647. PMCID: PMC5451078.
Article41. Miranda JP, Camões SP, Gaspar MM, Rodrigues JS, Carvalheiro M, Bárcia RN, Cruz P, Cruz H, Simões S, Santos JM. 2019; The secretome derived from 3D-cultured umbilical cord tissue MSCs counteracts manifestations typifying rheumatoid arthritis. Front Immunol. 10:18. DOI: 10.3389/fimmu.2019.00018. PMID: 30804924. PMCID: PMC6370626.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Therapeutic Effects of Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells on Scleroderma
- The Significance of NOTCH Pathway in the Development of Fibrosis in Systemic Sclerosis
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Diagnosis of Systemic Scleroderma in a Patient with Leg Ulcer