Korean J Gastroenterol.
2021 Jul;78(1):9-23. 10.4166/kjg.2021.409.
Sex- and Gender-related Issues of Gut Microbiota in Gastrointestinal Tract Diseases
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2518745
- DOI: http://doi.org/10.4166/kjg.2021.409
Abstract
- The distribution of gut microbiota varies according to age and sex. Gut microbiota are known to contribute to gastrointestinal (GI) diseases such as irritable bowel syndrome, inflammatory bowel disease, and colon cancer; however, the exact etiology remains elusive. Sex hormone such as estrogen and testosterone control the microbiota mainly due to different effect on the immunity. In addition, the diet depending on gender also affect the gut microbiota. Furthermore, the metabolism of estrogen and androgen was reported to be related to the gut microbiome. However, there have been few comprehensive review articles regarding the effect of microbiota on GI diseases. In this review, the factors which affect the gut microbiota and the interplay of microbiota and GI diseases in terms of sex- and gender differences were briefly summarized.
Keyword
Figure
Reference
-
1. Osadchiy V, Martin CR, Mayer EA. 2019; The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 17:322–332. DOI: 10.1016/j.cgh.2018.10.002. PMID: 30292888. PMCID: PMC6999848.
Article2. Kim YS, Unno T, Kim BY, Park MS. 2020; Sex differences in gut microbiota. World J Mens Health. 38:48–60. DOI: 10.5534/wjmh.190009. PMID: 30929328. PMCID: PMC6920072.
Article3. Kwa M, Plottel CS, Blaser MJ, Adams S. 2016; The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 108:djw029.4. Seo AY, Kim N, Oh DH. 2013; Abdominal bloating: pathophysiology and treatment. J Neurogastroenterol Motil. 19:433–453. DOI: 10.5056/jnm.2013.19.4.433. PMID: 24199004. PMCID: PMC3816178.
Article5. De Filippo C, Cavalieri D, Di Paola M, et al. 2010; Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 107:14691–14696. DOI: 10.1073/pnas.1005963107. PMID: 20679230. PMCID: PMC2930426.
Article6. Lee SM, Kim N, Yoon H, Nam RH, Lee DH. 2018; Microbial changes and host response in F344 rat colon depending on sex and age following a high-fat diet. Front Microbiol. 9:2236. DOI: 10.3389/fmicb.2018.02236. PMID: 30298061. PMCID: PMC6160749.
Article7. Foley KP, Zlitni S, Denou E, et al. 2018; Long term but not short term exposure to obesity related microbiota promotes host insulin resistance. Nat Commun. 9:4681. DOI: 10.1038/s41467-018-07146-5. PMID: 30409977. PMCID: PMC6224578.
Article8. Lee HS, Cho YH, Park J, Shin HR, Sung MK. 2013; Dietary intake of phytonutrients in relation to fruit and vegetable consumption in Korea. J Acad Nutr Diet. 113:1194–1199. DOI: 10.1016/j.jand.2013.04.022. PMID: 23830325.
Article9. Regu GM, Kim H, Kim YJ, et al. 2017; Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30-75 years using data from the fourth and fifth Korean national health and nutrition examination surveys (2008-2011). Nutrients. 9:1025. DOI: 10.3390/nu9091025. PMID: 28926945. PMCID: PMC5622785.
Article10. Jašarević E, Morrison KE, Bale TL. 2016; Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci. 371:20150122. DOI: 10.1098/rstb.2015.0122. PMID: 26833840. PMCID: PMC4785905.
Article11. Bäckhed F, Roswall J, Peng Y, et al. 2015; Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 17:852. DOI: 10.1016/j.chom.2015.05.012. PMID: 26308884.
Article12. Dominguez-Bello MG, Costello EK, Contreras M, et al. 2010; Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 107:11971–11975. DOI: 10.1073/pnas.1002601107. PMID: 20566857. PMCID: PMC2900693.
Article13. Blustein J, Attina T, Liu M, et al. 2013; Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond). 37:900–906. DOI: 10.1038/ijo.2013.49. PMID: 23670220. PMCID: PMC5007946.
Article14. Roduit C, Scholtens S, de Jongste JC, et al. 2009; Asthma at 8 years of age in children born by caesarean section. Thorax. 64:107–113. DOI: 10.1136/thx.2008.100875. PMID: 19052046.
Article15. Renz-Polster H, David MR, Buist AS, et al. 2005; Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 35:1466–1472. DOI: 10.1111/j.1365-2222.2005.02356.x. PMID: 16297144.
Article16. Curran EA, O'Neill SM, Cryan JF, et al. 2015; Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 56:500–508. DOI: 10.1111/jcpp.12351. PMID: 25348074.
Article17. Curran EA, Cryan JF, Kenny LC, Dinan TG, Kearney PM, Khashan AS. 2016; Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. J Autism Dev Disord. 46:603–614. DOI: 10.1007/s10803-015-2616-1. PMID: 26412364.
Article18. Jašarević E, Howerton CL, Howard CD, Bale TL. 2015; Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 156:3265–3276. DOI: 10.1210/en.2015-1177. PMID: 26079804. PMCID: PMC4541625.
Article19. Kunji ER, Mierau I, Hagting A, Poolman B, Konings WN. 1996; The proteolytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek. 70:187–221. DOI: 10.1007/BF00395933. PMID: 8879407.
Article20. Jiang T, Savaiano DA. 1997; In vitro lactose fermentation by human colonic bacteria is modified by Lactobacillus acidophilus supplementation. J Nutr. 127:1489–1495. DOI: 10.1093/jn/127.8.1489. PMID: 9237942.
Article21. Soergel KH. 1994; Colonic fermentation: metabolic and clinical implications. Clin Investig. 72:742–748. DOI: 10.1007/BF00180540. PMID: 7865976.
Article22. McDonald JW, Johnston MV. 1990; Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 15:41–70. DOI: 10.1016/0165-0173(90)90011-C.
Article23. McDonald JW, Johnston MV. 1993; Excitatory amino acid neurotoxicity in the developing brain. NIDA Res Monogr. 133:185–205. DOI: 10.1037/e495962006-010.
Article24. Hollister EB, Riehle K, Luna RA, et al. 2015; Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 3:36. DOI: 10.1186/s40168-015-0101-x. PMID: 26306392. PMCID: PMC4550057.
Article25. Sisk CL, Foster DL. 2004; The neural basis of puberty and adolescence. Nat Neurosci. 7:1040–1047. DOI: 10.1038/nn1326. PMID: 15452575.
Article26. Yurkovetskiy L, Burrows M, Khan AA, et al. 2013; Gender bias in autoimmunity is influenced by microbiota. Immunity. 39:400–412. DOI: 10.1016/j.immuni.2013.08.013. PMID: 23973225. PMCID: PMC3822899.
Article27. Markle JG, Frank DN, Mortin-Toth S, et al. 2013; Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 339:1084–1088. DOI: 10.1126/science.1233521. PMID: 23328391.
Article28. Spor A, Koren O, Ley R. 2011; Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 9:279–290. DOI: 10.1038/nrmicro2540. PMID: 21407244.
Article29. Mueller S, Saunier K, Hanisch C, et al. 2006; Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 72:1027–1033. DOI: 10.1128/AEM.72.2.1027-1033.2006. PMID: 16461645. PMCID: PMC1392899.
Article30. Ober C, Loisel DA, Gilad Y. 2008; Sex-specific genetic architecture of human disease. Nat Rev Genet. 9:911–922. DOI: 10.1038/nrg2415. PMID: 19002143. PMCID: PMC2694620.
Article31. Viña J, Borrás C, Gambini J, Sastre J, Pallardó FV. 2005; Why females live longer than males: control of longevity by sex hormones. Sci Aging Knowledge Environ. 2005:pe17. DOI: 10.1126/sageke.2005.23.pe17. PMID: 15944465.
Article32. Claesson MJ, Cusack S, O'Sullivan O, et al. 2011; Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 108 Suppl 1:4586–4591. DOI: 10.1073/pnas.1000097107. PMID: 20571116. PMCID: PMC3063589.
Article33. Claesson MJ, Jeffery IB, Conde S, et al. 2012; Gut microbiota composition correlates with diet and health in the elderly. Nature. 488:178–184. DOI: 10.1038/nature11319. PMID: 22797518.
Article34. Rampelli S, Candela M, Turroni S, et al. 2013; Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY). 5:902–912. DOI: 10.18632/aging.100623. PMID: 24334635. PMCID: PMC3883706.
Article35. Biagi E, Nylund L, Candela M, et al. 2010; Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 5:e10667. DOI: 10.1371/journal.pone.0010667. PMID: 20498852. PMCID: PMC2871786.
Article36. Bischoff SC. 2016; Microbiota and aging. Curr Opin Clin Nutr Metab Care. 19:26–30. DOI: 10.1097/MCO.0000000000000242. PMID: 26560527.37. Salles N. 2007; Basic mechanisms of the aging gastrointestinal tract. Dig Dis. 25:112–117. DOI: 10.1159/000099474. PMID: 17468545.
Article38. Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. 2016; Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 65:57–62. DOI: 10.1136/gutjnl-2015-309618. PMID: 26069274. PMCID: PMC4717365.
Article39. Parthasarathy G, Chen J, Chen X, et al. 2016; Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology. 150:367–379.e1. DOI: 10.1053/j.gastro.2015.10.005. PMID: 26460205. PMCID: PMC4727996.
Article40. Enck P, Zimmermann K, Rusch K, Schwiertz A, Klosterhalfen S, Frick JS. 2009; The effects of ageing on the colonic bacterial microflora in adults. Z Gastroenterol. 47:653–658. DOI: 10.1055/s-0028-1109055. PMID: 19606407.
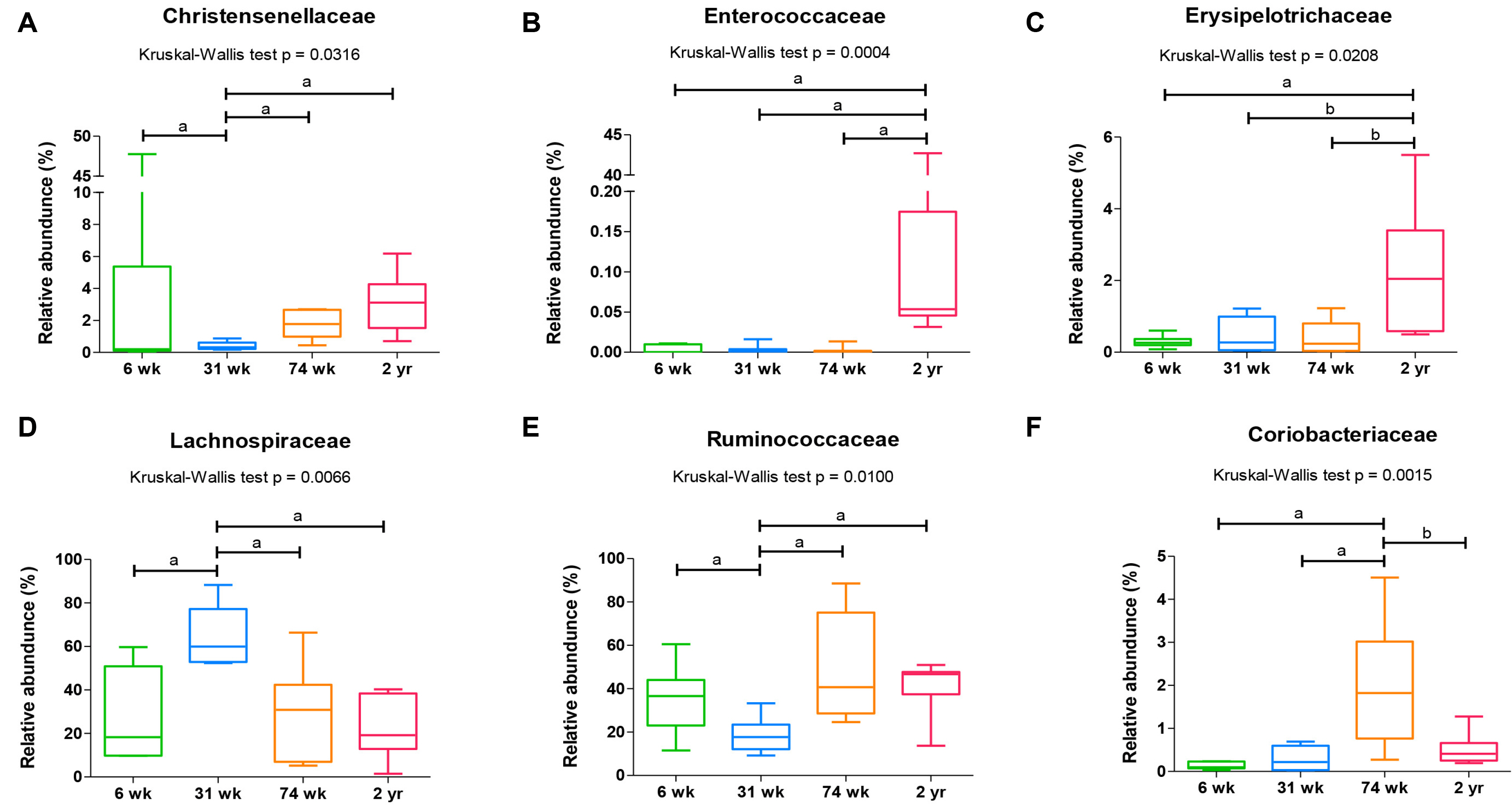
Article41. Choi SI, Son JH, Kim N, et al. 2021; Changes in cecal microbiota and short-chain fatty acid during lifespan of the rat. J Neurogastroenterol Motil. 27:134–146. DOI: 10.5056/jnm20148. PMID: 33380558. PMCID: PMC7786083.
Article42. Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, et al. 2018; Influence of gender and menopausal status on gut microbiota. Maturitas. 116:43–53. DOI: 10.1016/j.maturitas.2018.07.008. PMID: 30244778.
Article43. Sender R, Fuchs S, Milo R. 2016; Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14:e1002533. DOI: 10.1371/journal.pbio.1002533. PMID: 27541692. PMCID: PMC4991899.
Article44. Roger LC, McCartney AL. 2010; Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology (Reading). 156:3317–3328. DOI: 10.1099/mic.0.041913-0. PMID: 20829292.
Article45. Vulevic J, Juric A, Tzortzis G, Gibson GR. 2013; A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J Nutr. 143:324–331. DOI: 10.3945/jn.112.166132. PMID: 23303873.
Article46. Davy KP, Seals DR. 1994; Total blood volume in healthy young and older men. J Appl Physiol. 76:2059–2062. DOI: 10.1152/jappl.1994.76.5.2059. PMID: 8063668.
Article47. Retzlaff JA, Tauxe WN, Kiely JM, Stroebel CF. 1969; Erythrocyte volume, plasma volume, and lean body mass in adult men and women. Blood. 33:649–661. DOI: 10.1182/blood.V33.5.649.649. PMID: 5779153.
Article48. Young JF, Luecke RH, Pearce BA, et al. 2009; Human organ/tissue growth algorithms that include obese individuals and black/white population organ weight similarities from autopsy data. J Toxicol Environ Health A. 72:527–540. DOI: 10.1080/15287390802647203. PMID: 19267313.
Article49. Sinha T, Vich Vila A, Garmaeva S, et al. 2019; Analysis of 1135 gut meta-genomes identifies sex-specific resistome profiles. Gut Microbes. 10:358–366. DOI: 10.1080/19490976.2018.1528822. PMID: 30373468. PMCID: PMC6546312.
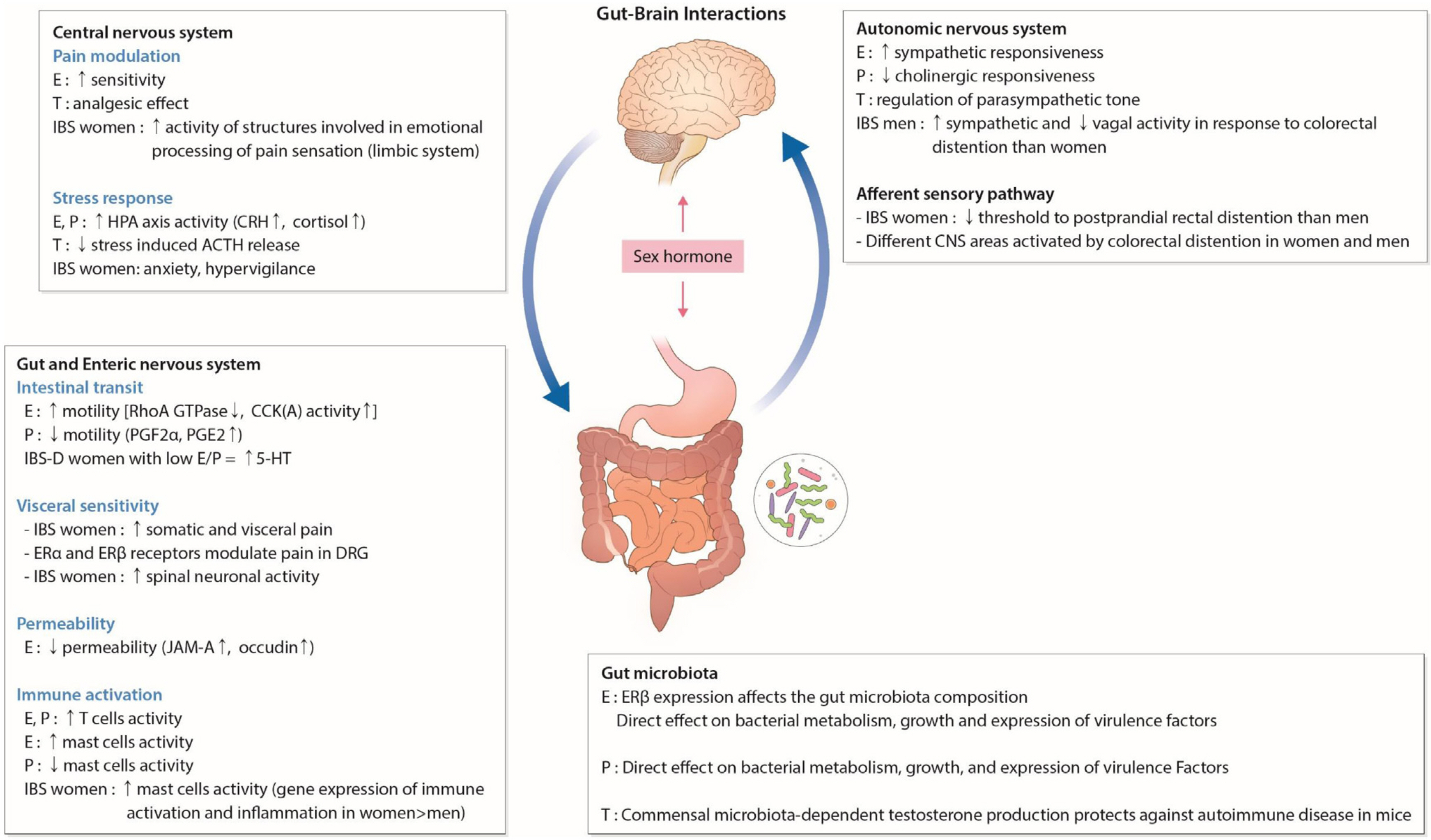
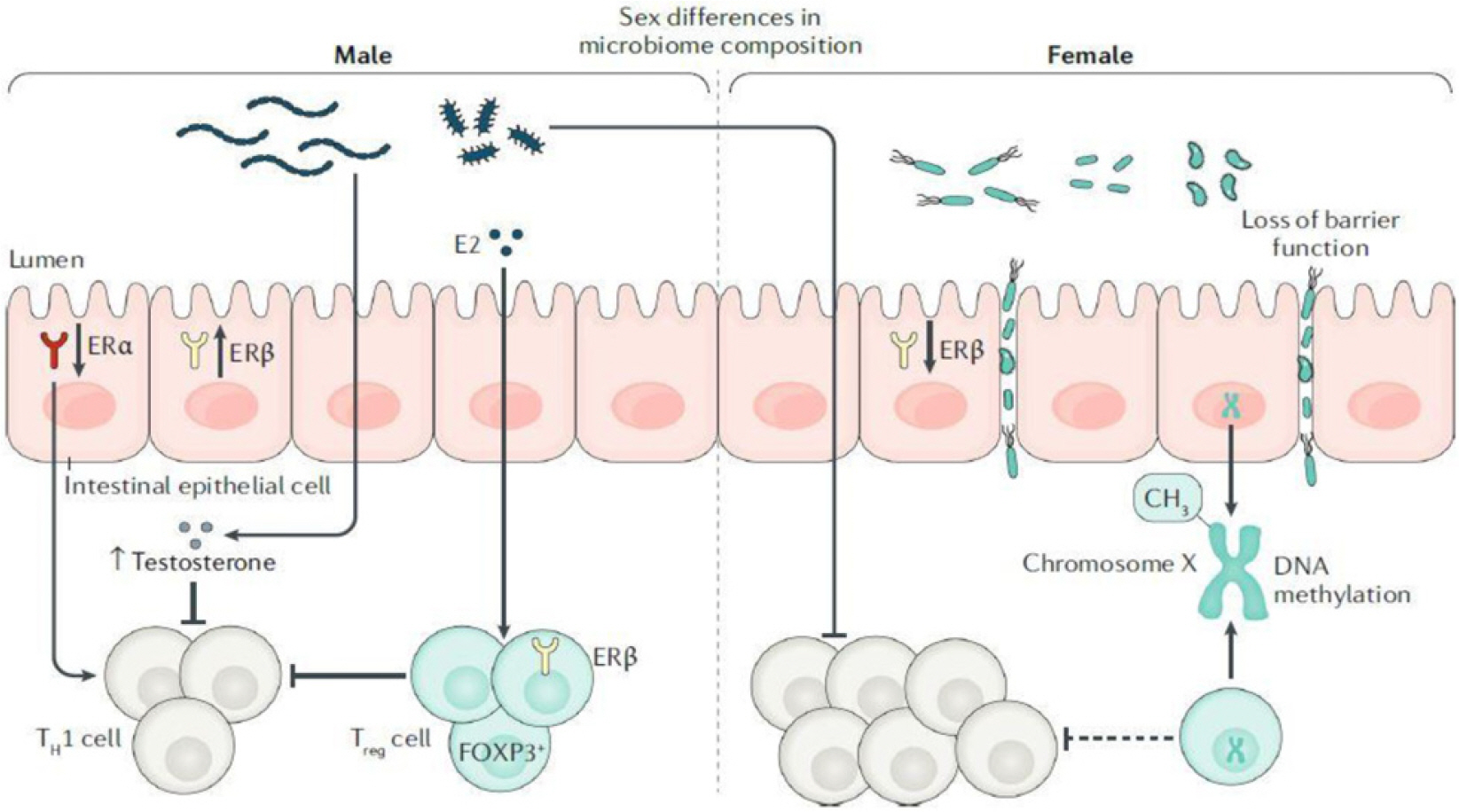
Article50. Yoon K, Kim N. 2021; Roles of sex hormones and gender in the gut microbiota. J Neurogastroenterol Motil. 27:314–325. DOI: 10.5056/jnm20208. PMID: 33762473. PMCID: PMC8266488.
Article51. Gomez A, Luckey D, Taneja V. 2015; The gut microbiome in autoimmunity: sex matters. Clin Immunol. 159:154–162. DOI: 10.1016/j.clim.2015.04.016. PMID: 25956531. PMCID: PMC4560596.
Article52. Menon R, Watson SE, Thomas LN, et al. 2013; Diet complexity and estrogen receptor β status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol. 79:5763–5773. DOI: 10.1128/AEM.01182-13. PMID: 23872567. PMCID: PMC3754184.
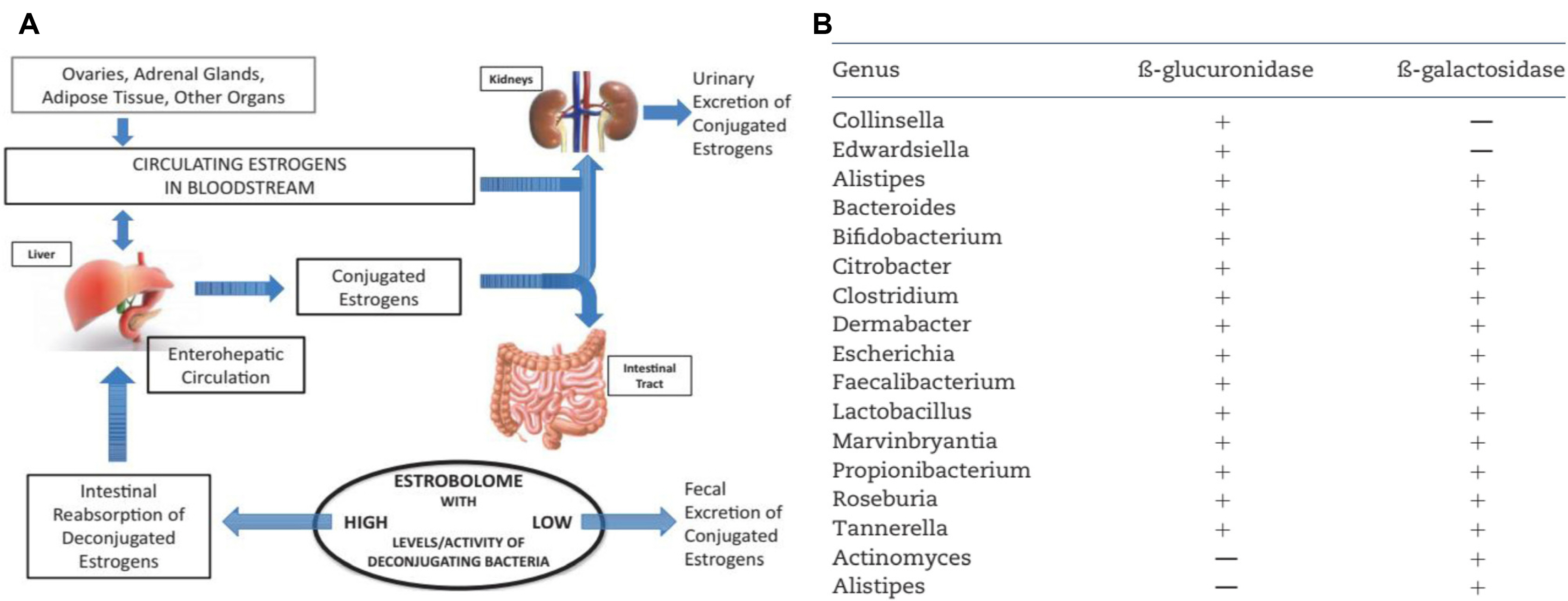
Article53. Flores R, Shi J, Fuhrman B, et al. 2012; Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 10:253. DOI: 10.1186/1479-5876-10-253. PMID: 23259758. PMCID: PMC3552825.
Article54. Colldén H, Landin A, Wallenius V, et al. 2019; The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol Endocrinol Metab. 317:E1182–E1192. DOI: 10.1152/ajpendo.00338.2019. PMID: 31689143. PMCID: PMC6962501.
Article55. Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. 2019; Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 170:192–201. DOI: 10.1016/j.resmic.2019.03.003. PMID: 30940469.
Article56. Drossman DA, Whitehead WE, Camilleri M. 1997; Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology. 112:2120–2137. DOI: 10.1053/gast.1997.v112.agast972120. PMID: 9178709.
Article57. Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. 2002; Utility of the Rome I and Rome II criteria for irritable bowel syndrome in U.S. women. Am J Gastroenterol. 97:2803–2811. DOI: 10.1111/j.1572-0241.2002.07026.x. PMID: 12425552.
Article58. Sandler RS. 1990; Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 99:409–415. DOI: 10.1016/0016-5085(90)91023-Y.
Article59. Whitehead WE, Palsson O, Jones KR. 2002; Systematic review of the co-morbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 122:1140–1156. DOI: 10.1053/gast.2002.32392. PMID: 11910364.
Article60. Azpiroz F, Dapoigny M, Pace F, et al. 2000; Nongastrointestinal disorders in the irritable bowel syndrome. Digestion. 62:66–72. DOI: 10.1159/000007780. PMID: 10899728.
Article61. Ahlawat SK, Cuddihy MT, Locke GR 3rd. 2006; Gender-related differences in dyspepsia: a qualitative systematic review. Gend Med. 3:31–42. DOI: 10.1016/S1550-8579(06)80192-0.
Article62. Kim YS, Kim N. 2018; Sex-gender differences in irritable bowel syndrome. J Neurogastroenterol Motil. 24:544–558. DOI: 10.5056/jnm18082. PMID: 30347934. PMCID: PMC6175559.
Article63. Choghakhori R, Abbasnezhad A, Amani R, Alipour M. 2017; Sex-related differences in clinical symptoms, quality of life, and biochemical factors in irritable bowel syndrome. Dig Dis Sci. 62:1550–1560. DOI: 10.1007/s10620-017-4554-6. PMID: 28374085.
Article64. Choi YJ, Kim N, Yoon H, et al. 2017; Overlap between irritable bowel syndrome and functional dyspepsia including subtype analyses. J Gastroenterol Hepatol. 32:1553–1561. DOI: 10.1111/jgh.13756. PMID: 28160607.
Article65. Lampe JW, Fredstrom SB, Slavin JL, Potter JD. 1993; Sex differences in colonic function: a randomised trial. Gut. 34:531–536. DOI: 10.1136/gut.34.4.531. PMID: 8387940. PMCID: PMC1374316.
Article66. Aloisi AM. 2003; Gonadal hormones and sex differences in pain reactivity. Clin J Pain. 19:168–174. DOI: 10.1097/00002508-200305000-00004. PMID: 12792555.
Article67. Cairns BE, Gazerani P. 2009; Sex-related differences in pain. Maturitas. 63:292–296. DOI: 10.1016/j.maturitas.2009.06.004. PMID: 19595525.
Article68. Houghton LA, Jackson NA, Whorwell PJ, Morris J. 2000; Do male sex hormones protect from irritable bowel syndrome? Am J Gastroenterol. 95:2296–2300. DOI: 10.1111/j.1572-0241.2000.02314.x. PMID: 11007231.
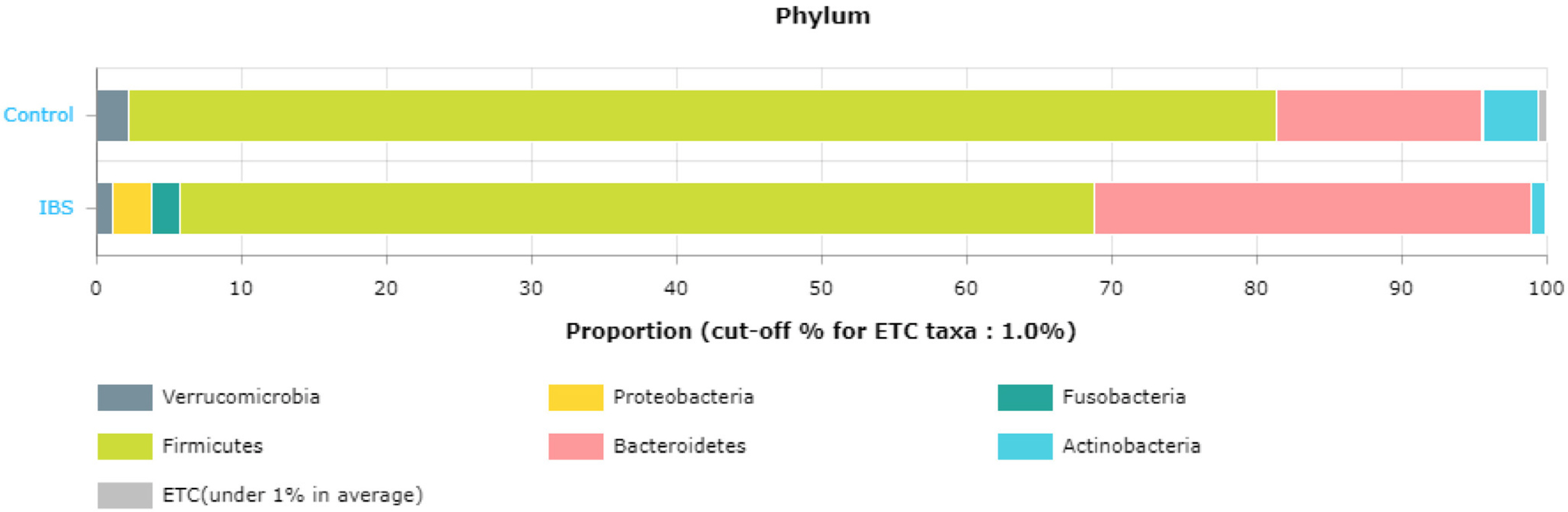
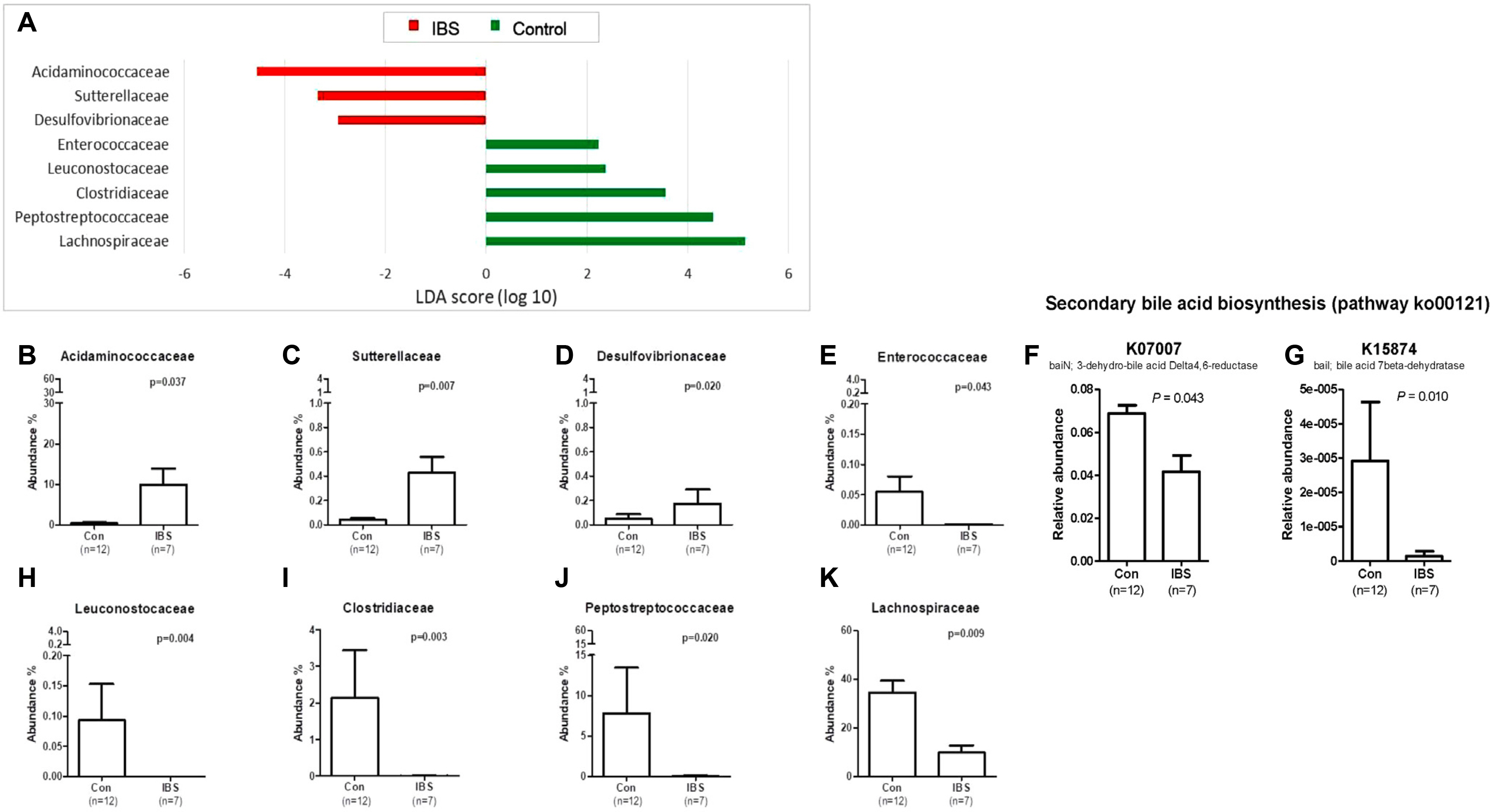
Article69. Lee SM, Kim N, Yoon H, et al. 2021; Compositional and functional changes in the gut microbiota in irritable bowel syndrome patients. Gut Liver. 15:253–261. DOI: 10.5009/gnl19379. PMID: 32457278. PMCID: PMC7960967.
Article70. Distrutti E, Monaldi L, Ricci P, Fiorucci S. 2016; Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol. 22:2219–2241. DOI: 10.3748/wjg.v22.i7.2219. PMID: 26900286. PMCID: PMC4734998.
Article71. Ait-Belgnaoui A, Payard I, Rolland C, et al. 2018; Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J Neurogastroenterol Motil. 24:138–146. DOI: 10.5056/jnm16167. PMID: 29291614. PMCID: PMC5753912.72. Pigrau M, Rodiño-Janeiro BK, Casado-Bedmar M, et al. 2016; The joint power of sex and stress to modulate brain-gut-microbiota axis and intestinal barrier homeostasis: implications for irritable bowel syndrome. Neurogastroenterol Motil. 28:463–486. DOI: 10.1111/nmo.12717. PMID: 26556786.
Article73. Braus NA, Elliott DE. 2009; Advances in the pathogenesis and treatment of IBD. Clin Immunol. 132:1–9. DOI: 10.1016/j.clim.2009.02.006. PMID: 19321388. PMCID: PMC2693446.
Article74. McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. 2009; Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 15:100–113. DOI: 10.1002/ibd.20539. PMID: 18623167.
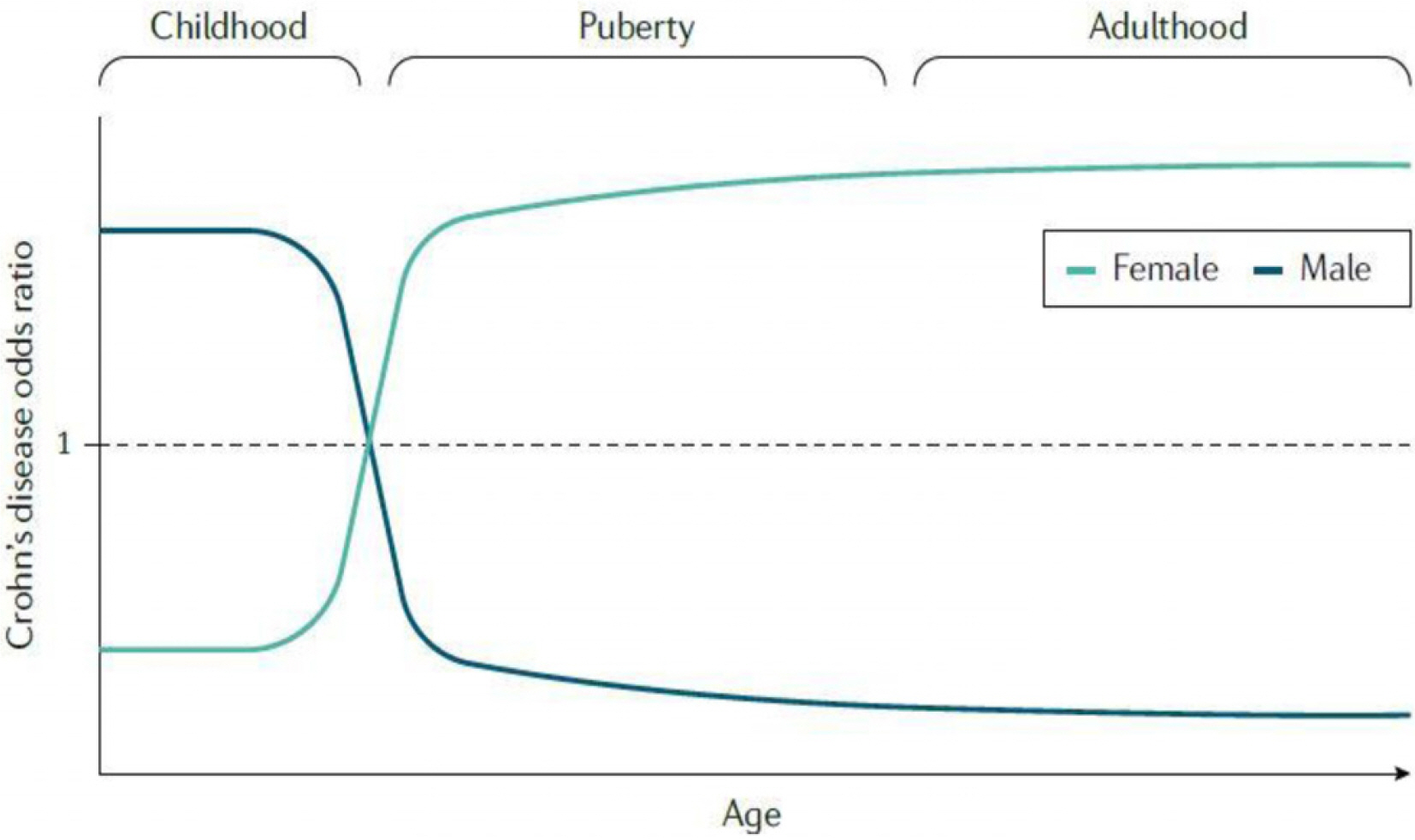
Article75. Goodman WA, Erkkila IP, Pizarro TT. 2020; Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 17:740–754. DOI: 10.1038/s41575-020-0354-0. PMID: 32901108.
Article76. Shah SC, Khalili H, Gower-Rousseau C, et al. 2018; Sex-based differences in incidence of inflammatory bowel diseases-pooled analysis of population-based studies from Western countries. Gastroenterology. 155:1079–1089.e3.
Article77. Song CH, Kim N, Sohn SH, et al. 2018; Effects of 17β-estradiol on colonic permeability and inflammation in an azoxymethane/dextran sulfate sodium-induced colitis mouse model. Gut Liver. 12:682–693. DOI: 10.5009/gnl18221. PMID: 30400733. PMCID: PMC6254630.78. Son HJ, Kim N, Song CH, Lee SM, Lee HN, Surh YJ. 2020; 17β-Estradiol reduces inflammation and modulates antioxidant enzymes in colonic epithelial cells. Korean J Intern Med. 35:310–319. DOI: 10.3904/kjim.2018.098. PMID: 30336658. PMCID: PMC7061017.
Article79. Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. 2008; The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 103:2394–2400. DOI: 10.1111/j.1572-0241.2008.02064.x. PMID: 18684177.
Article80. Ortizo R, Lee SY, Nguyen ET, Jamal MM, Bechtold MM, Nguyen DL. 2017; Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur J Gastroenterol Hepatol. 29:1064–1070. DOI: 10.1097/MEG.0000000000000915. PMID: 28542115.81. Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. 2005; Increased risk of intestinal cancer in Crohn's disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 100:2724–2729. DOI: 10.1111/j.1572-0241.2005.00287.x. PMID: 16393226.
Article82. Saha S, Zhao YQ, Shah SA, et al. 2014; Menstrual cycle changes in women with inflammatory bowel disease: a study from the ocean state Crohn's and colitis area registry. Inflamm Bowel Dis. 20:534–540. DOI: 10.1097/01.MIB.0000441347.94451.cf. PMID: 24451220. PMCID: PMC4347838.83. Rustgi SD, Kayal M, Shah SC. 2020; Apr. 28. Sex-based differences in inflammatory bowel diseases: a review. Therap Adv Gastroenterol. [Epub ahead of print]. DOI: 10.1177/1756284820915043. PMID: 32523620. PMCID: PMC7236567.
Article84. Geuking MB, Köller Y, Rupp S, McCoy KD. 2014; The interplay between the gut microbiota and the immune system. Gut Microbes. 5:411–418. DOI: 10.4161/gmic.29330. PMID: 24922519. PMCID: PMC4153781.
Article85. Son HJ, Kim N, Song CH, et al. 2019; Sex-related alterations of gut microbiota in the C57BL/6 mouse model of inflammatory bowel disease. J Cancer Prev. 24:173–182. DOI: 10.15430/JCP.2019.24.3.173. PMID: 31624723. PMCID: PMC6786806.
Article86. Org E, Mehrabian M, Parks BW, et al. 2016; Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 7:313–322. DOI: 10.1080/19490976.2016.1203502. PMID: 27355107. PMCID: PMC4988450.
Article87. Song CH, Kim N, Nam RH, Choi SI, Lee HN, Surh YJ. 2020; 17β-Estradiol supplementation changes gut microbiota diversity in intact and colorectal cancer-induced ICR male mice. Sci Rep. 10:12283. DOI: 10.1038/s41598-020-69112-w. PMID: 32704056. PMCID: PMC7378548.
Article88. Kozik AJ, Nakatsu CH, Chun H, Jones-Hall YL. 2017; Age, sex, and TNF associated differences in the gut microbiota of mice and their impact on acute TNBS colitis. Exp Mol Pathol. 103:311–319. DOI: 10.1016/j.yexmp.2017.11.014. PMID: 29175304.
Article89. Lee SM, Kim N, Son HJ, et al. 2016; The effect of sex on the azoxymethane/dextran sulfate sodium-treated mice model of colon cancer. J Cancer Prev. 21:271–278. DOI: 10.15430/JCP.2016.21.4.271. PMID: 28053962. PMCID: PMC5207612.
Article90. Son HJ, Sohn SH, Kim N, et al. 2019; Effect of estradiol in an azoxymethane/dextran sulfate sodium-treated mouse model of colorectal cancer: implication for sex difference in colorectal cancer development. Cancer Res Treat. 51:632–648. DOI: 10.4143/crt.2018.060. PMID: 30064198. PMCID: PMC6473282.
Article91. Song CH, Kim N, Lee SM, et al. 2019; Effects of 17β-estradiol on color-ectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem Pharmacol. 164:139–151. DOI: 10.1016/j.bcp.2019.04.011. PMID: 30981879.92. Song CH, Kim N, Nam RH, et al. 2021; Testosterone strongly enhances azoxymethane/dextran sulfate sodium-induced colorectal cancer development in C57BL/6 mice. Am J Can Res. 11:3145–3162.93. Yoon K, Kim N. 2018; The effect of microbiota on colon carcinogenesis. J Cancer Prev. 23:117–125. DOI: 10.15430/JCP.2018.23.3.117. PMID: 30370256. PMCID: PMC6197845.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Roles of Sex Hormones and Gender in the Gut Microbiota
- Pathogenic role of the gut microbiota in gastrointestinal diseases
- Sex Differences in Gut Microbiota
- Revolutionizing gut health: exploring the role of gut microbiota and the potential of microbiome-based therapies in lower gastrointestinal diseases
- Gut Microbiota and Pancreatobiliary System