Cancer Res Treat.
2021 Jul;53(3):847-856. 10.4143/crt.2020.1060.
Prognostic Stratification of Patients with Burkitt Lymphoma Using Serum β2-microglobulin Levels
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Division of Hematology Oncology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2518409
- DOI: http://doi.org/10.4143/crt.2020.1060
Abstract
- Purpose
We aimed to investigate the prognostic value of serum β2-microglobulin for patients with Burkitt lymphoma (BL) and to propose a risk-stratifying classification system.
Materials and Methods
A prospective registry-based cohort study of BL patients treated with dose-intensive or effective dose-adjusted chemotherapies (n=81) was conducted. Survival outcomes were compared based on previously reported risk groups and/or serum β2-microglobulin levels. A risk-stratifying classification system incorporating serum β2-microglobulin levels was proposed and validated in an independent validation cohort (n=60).
Results
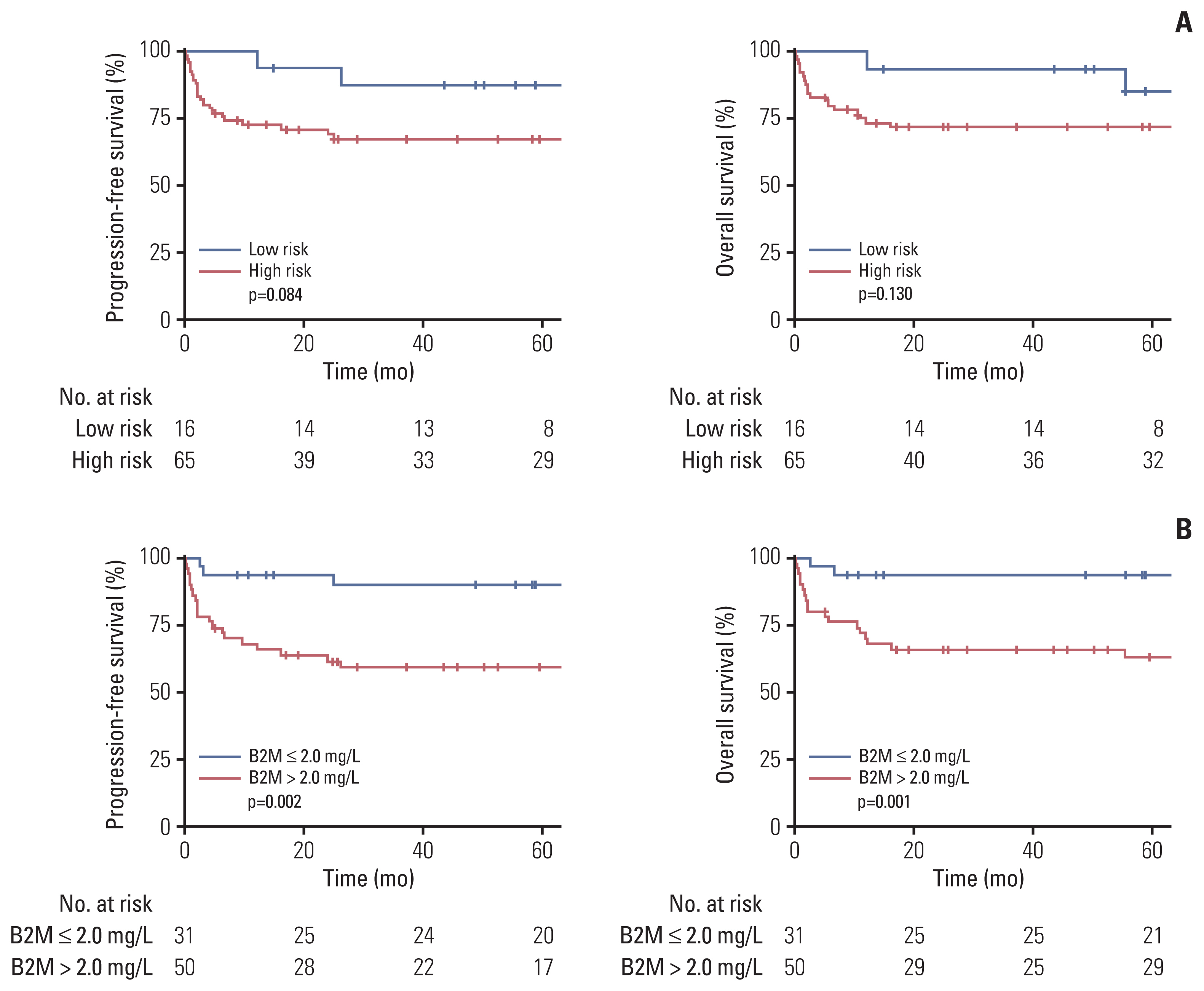
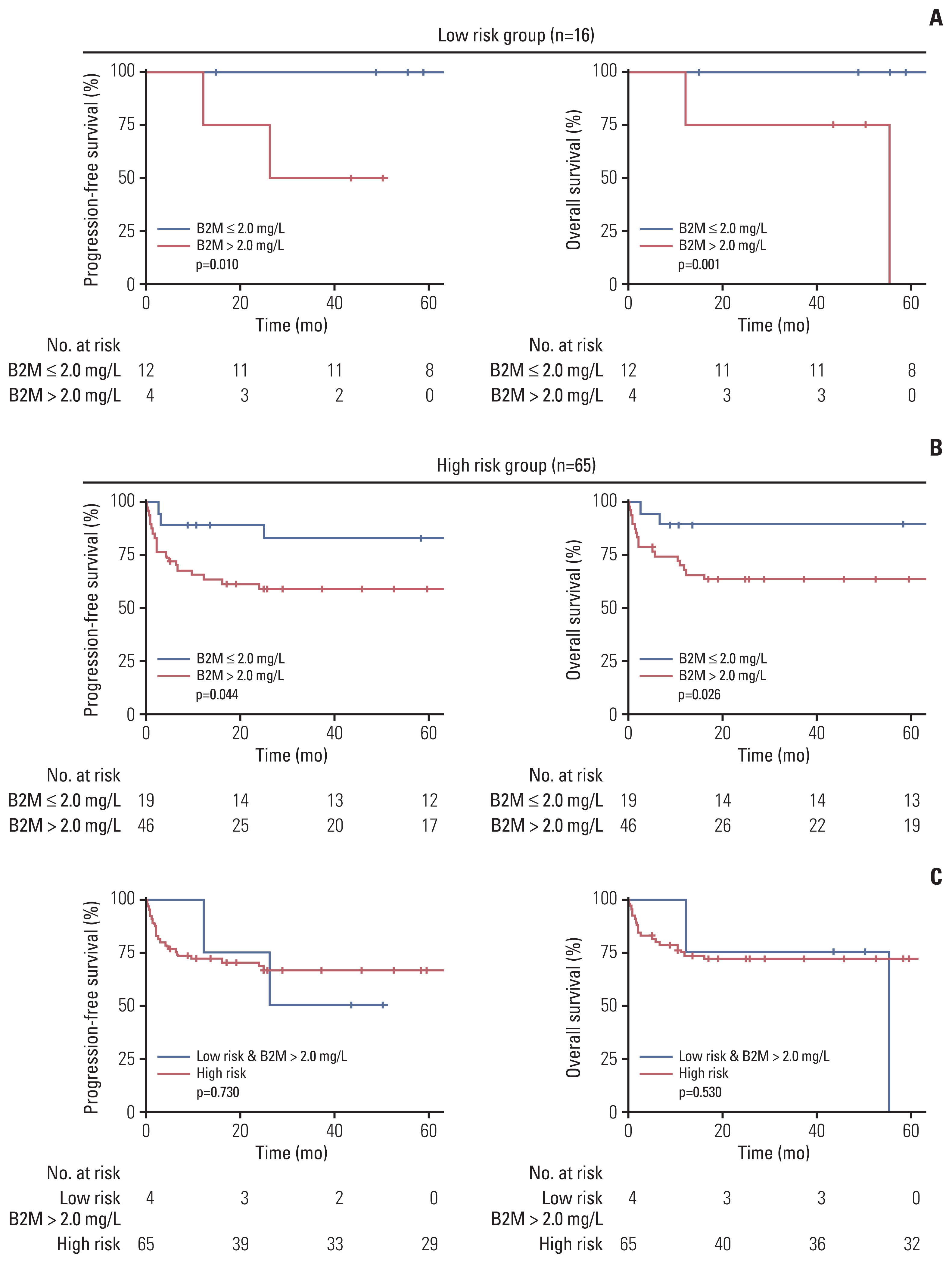
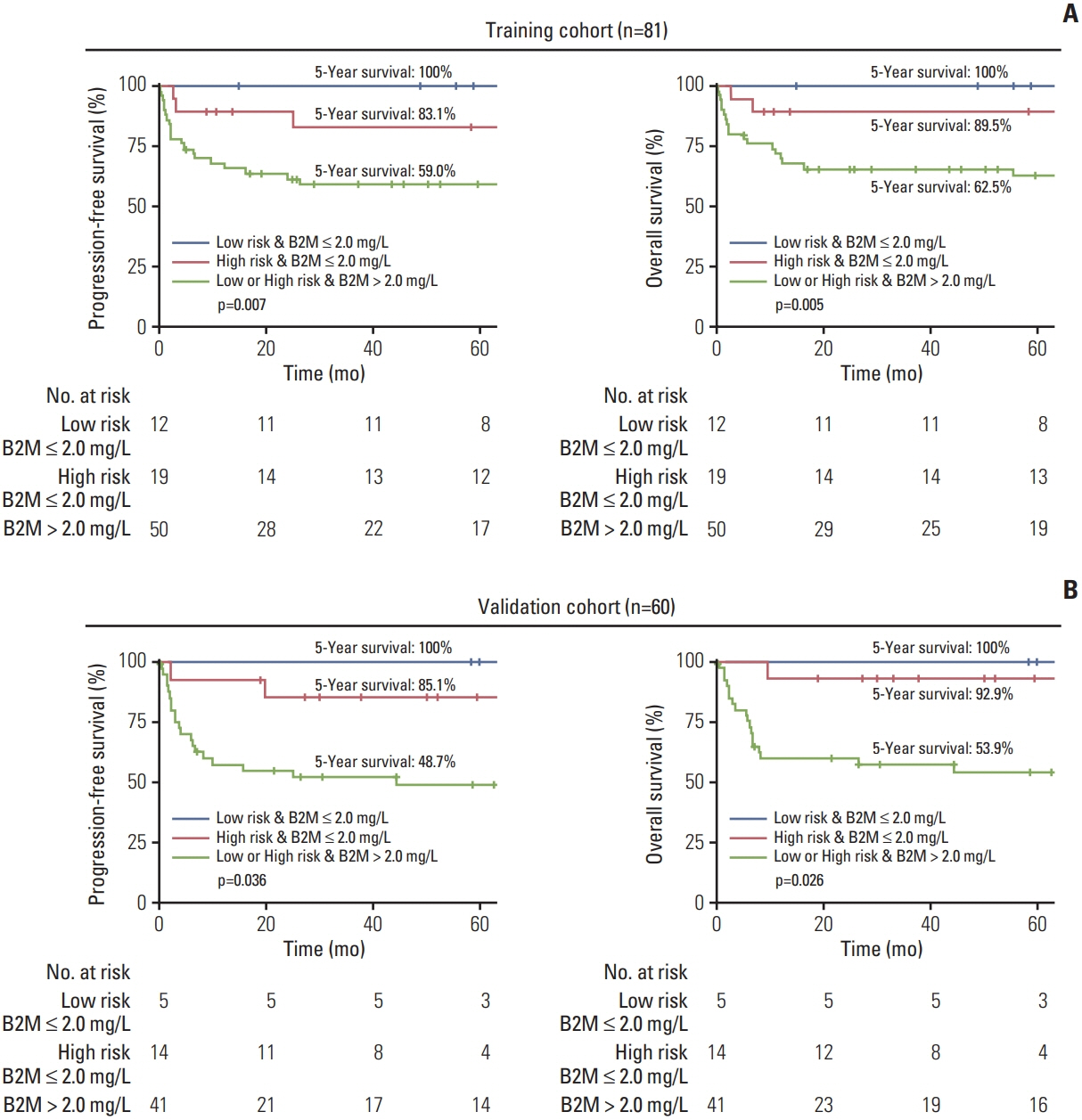
The median age was 47 years, and 57 patients (70.4%) were male. Patients with high serum β2-microglobulin levels (> 2 mg/L) had significantly worse progression-free survival (PFS) and overall survival (OS) (p < 0.01 for both). Serum β2-microglobulin levels further stratified patients in the low-risk and high-risk groups in terms of PFS (p=0.010 and p=0.044, respectively) and OS (p=0.014 and p=0.026, respectively). Multivariate analyses revealed that a high serum β2-microglobulin level (> 2 mg/L) was independently associated with a shorter PFS (hazards ratio [HR], 3.56; p=0.047) and OS (HR, 4.66; p=0.043). The new classification system incorporating the serum β2-microglobulin level allowed the stratification of patients into three distinct risk subgroups with 5-year OS rates of 100%, 89.5%, and 62.5%. In an independent cohort of BL, the system was validated by stratifying patients with different survival outcomes.
Conclusion
Serum β2-microglobulin level is an independent prognostic factor for BL patients. The proposed β2-microglobulin–based classification system could stratify patients with distinct survival outcomes, which may help define appropriate treatment approaches for individual patients.
Figure
Reference
-
References
1. Perkins AS, Friedberg JW. Burkitt lymphoma in adults. Hematology Am Soc Hematol Educ Program. 2008; 341–8.
Article2. Kalisz K, Alessandrino F, Beck R, Smith D, Kikano E, Ramaiya NH, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019; 10:56.
Article3. Hwang I, Go H, Jeon YK, Ko YH, Yoon DH, Suh C, et al. Expression of JL1 in Burkitt lymphoma is associated with improved overall survival. Virchows Arch. 2011; 459:353–9.
Article4. Jang SJ, Yoon DH, Kim S, Yoon S, Kim DY, Park CS, et al. A unique pattern of extranodal involvement in Korean adults with sporadic Burkitt lymphoma: a single center experience. Ann Hematol. 2012; 91:1917–22.
Article5. Wästerlid T, Jonsson B, Hagberg H, Jerkeman M. Population based study of prognostic factors and treatment in adult Burkitt lymphoma: a Swedish Lymphoma Registry study. Leuk Lymphoma. 2011; 52:2090–6.
Article6. Evens AM, Danilov AV, Jagadeesh D, Sperling AL, Kim SH, Vaca RA, et al. Burkitt lymphoma in the modern era: real world outcomes and prognostication across 30 US cancer centers. Blood. 2021; 137:374–386.7. Jakobsen LH, Ellin F, Smeland KB, Wasterlid T, Christensen JH, Jorgensen JM, et al. Minimal relapse risk and early normalization of survival for patients with Burkitt lymphoma treated with intensive immunochemotherapy: an international study of 264 real-world patients. Br J Haematol. 2020; 189:661–71.
Article8. Castillo JJ, Winer ES, Olszewski AJ. Population-based prognostic factors for survival in patients with Burkitt lymphoma: an analysis from the Surveillance, Epidemiology, and End Results database. Cancer. 2013; 119:3672–9.
Article9. Sweetenham JW, Pearce R, Taghipour G, Blaise D, Gisselbrecht C, Goldstone AH. Adult Burkitt’s and Burkitt-like non-Hodgkin’s lymphoma: outcome for patients treated with high-dose therapy and autologous stem-cell transplantation in first remission or at relapse: results from the European Group for Blood and Marrow Transplantation. J Clin Oncol. 1996; 14:2465–72.10. Mead GM, Barrans SL, Qian W, Walewski J, Radford JA, Wolf M, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood. 2008; 112:2248–60.
Article11. Roschewski M, Dunleavy K, Abramson JS, Powell BL, Link BK, Patel P, et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Bur-kitt lymphoma. J Clin Oncol. 2020; 38:2519–29.
Article12. Chronowski GM, Wilder RB, Tucker SL, Ha CS, Sarris AH, Hagemeister FB, et al. An elevated serum beta-2-microglobulin level is an adverse prognostic factor for overall survival in patients with early-stage Hodgkin disease. Cancer. 2002; 95:2534–8.
Article13. Yoo C, Yoon DH, Jo JC, Yoon S, Kim S, Lee BJ, et al. Prognostic impact of beta-2 microglobulin in patients with extranodal natural killer/T cell lymphoma. Ann Hematol. 2014; 93:995–1000.
Article14. Yoo C, Yoon DH, Yoon S, Kim S, Huh J, Park CJ, et al. Prognostic impact of beta(2)-microglobulin in patients with non-gastric mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma. 2015; 56:688–93.15. Johnson PW, Whelan J, Longhurst S, Stepniewska K, Matthews J, Amess J, et al. Beta-2 microglobulin: a prognostic factor in diffuse aggressive non-Hodgkin’s lymphomas. Br J Cancer. 1993; 67:792–7.16. Lee EJ, Petroni GR, Schiffer CA, Freter CE, Johnson JL, Barcos M, et al. Brief-duration high-intensity chemotherapy for patients with small noncleaved-cell lymphoma or FAB L3 acute lymphocytic leukemia: results of cancer and leukemia group B study 9251. J Clin Oncol. 2001; 19:4014–22.
Article17. Thomas DA, Faderl S, O’Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006; 106:1569–80.
Article18. Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013; 369:1915–25.
Article19. Choi MK, Jun HJ, Lee SY, Kim KH, Lim DH, Kim K, et al. Treatment outcome of adult patients with Burkitt lymphoma: results using the LMB protocol in Korea. Ann Hematol. 2009; 88:1099–106.
Article20. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–68.
Article21. Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009; 27:4555–62.
Article22. Lopez-Guillermo A, Colomo L, Jimenez M, Bosch F, Villamor N, Arenillas L, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005; 23:2797–804.23. Vassilakopoulos TP, Nadali G, Angelopoulou MK, Siakantaris MP, Dimopoulou MN, Kontopidou FN, et al. The prognostic significance of beta(2)-microglobulin in patients with Hodgkin’s lymphoma. Haematologica. 2002; 87:701–8.24. Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015; 33:2863–9.
Article25. Constantinides IP, Pathouli C, Karvountzis G, Papadopoulos P, Varvoutsi-Constantinides M, Eliakis P, et al. Serum beta 2 microglobulin in malignant lymphoproliferative disorders. Cancer. 1985; 55:2384–9.26. Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Pescosta N, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002; 13:1264–74.
Article27. Rizzieri DA, Johnson JL, Niedzwiecki D, Lee EJ, Vardiman JW, Powell BL, et al. Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: final results of Cancer and Leukemia Group B Study 9251. Cancer. 2004; 100:1438–48.28. Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001; 93:618–29.
Article