Endocrinol Metab.
2021 Jun;36(3):544-552. 10.3803/EnM.2021.301.
Long-Term Treatment of Postmenopausal Osteoporosis
- Affiliations
-
- 1Division of Rheumatology, Department of Medicine, Laval University, Quebec City, QC, Canada
- KMID: 2517643
- DOI: http://doi.org/10.3803/EnM.2021.301
Abstract
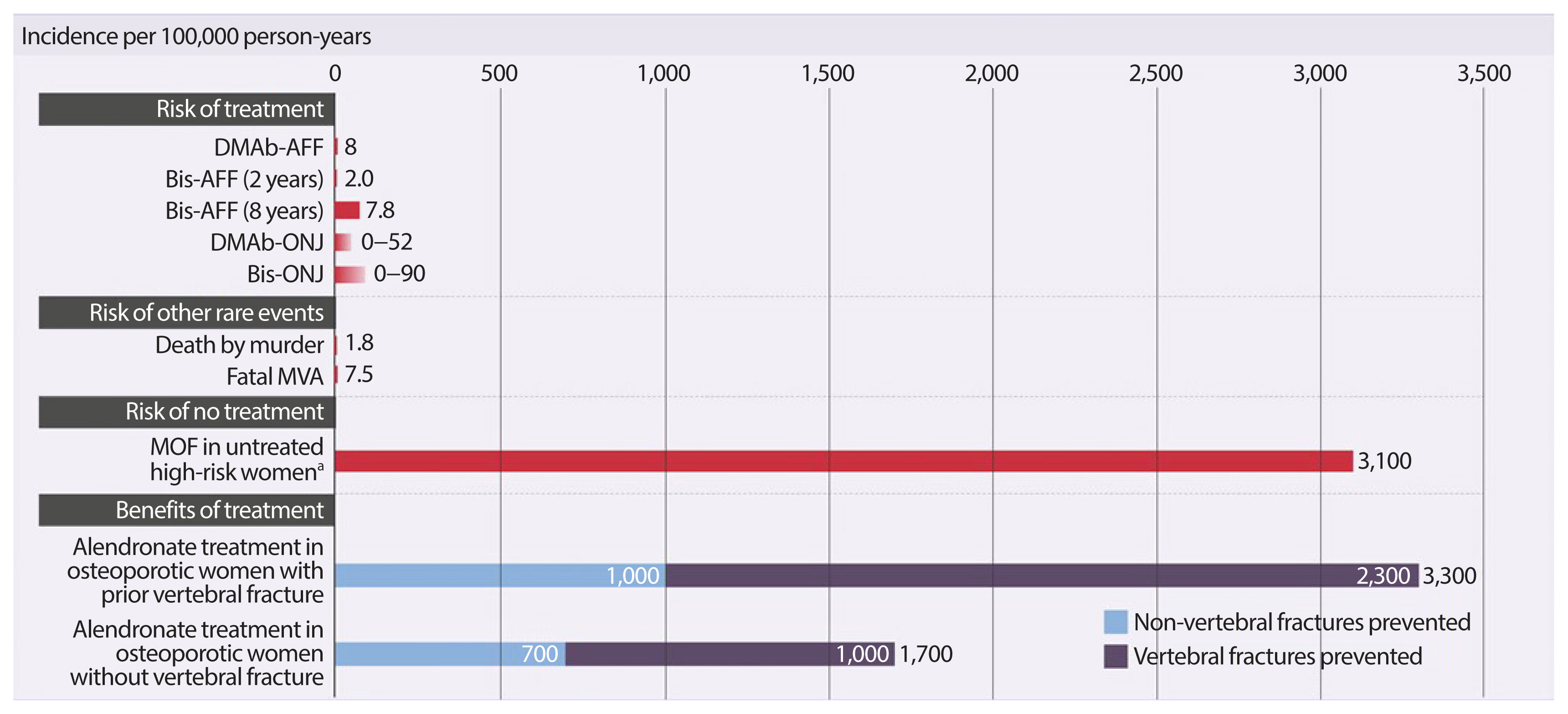
- Osteoporosis is an incurable chronic condition, like heart disease, diabetes, or hypertension. A large gap currently exists in the primary prevention of fractures, and studies show that an estimated 80% to 90% of adults do not receive appropriate osteoporosis management even in the secondary prevention setting. Case finding strategies have been developed and effective pharmacological interventions are available. This publication addresses how best to use the pharmacological options available for postmenopausal osteoporosis to provide lifelong fracture protection in patients at high and very high risk of fracture. The benefit of osteoporosis therapies far outweighs the rare risks.
Figure
Cited by 1 articles
-
Changes in Serum Dickkopf-1, RANK Ligand, Osteoprotegerin, and Bone Mineral Density after Allogeneic Hematopoietic Stem Cell Transplantation Treatment
Eunhee Jang, Jeonghoon Ha, Ki-Hyun Baek, Moo Il Kang
Endocrinol Metab. 2021;36(6):1211-1218. doi: 10.3803/EnM.2021.1248.
Reference
-
1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285:785–95.2. Bessette L, Ste-Marie LG, Jean S, Davison KS, Beaulieu M, Baranci M, et al. The care gap in diagnosis and treatment of women with a fragility fracture. Osteoporos Int. 2008; 19:79–86.
Article3. Adachi JD, Loannidis G, Berger C, Joseph L, Papaioannou A, Pickard L, et al. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int. 2001; 12:903–8.
Article4. Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES. Impact of recent fracture on health-related quality of life in postmenopausal women. J Bone Miner Res. 2006; 21:809–16.
Article5. Brown JP, Adachi JD, Schemitsch E, Tarride JE, Brown V, Bell A, et al. Mortality in older adults following a fragility fracture: real-world retrospective matched-cohort study in Ontario. BMC Musculoskelet Disord. 2021; 22:105.
Article6. Tarride JE, Adachi JD, Brown JP, Schemitsch E, Slatkovska L, Burke N. Incremental costs of fragility fractures: a population-based matched-cohort study from Ontario, Canada. Osteoporos Int. 2021. Feb. 18. [Epub]. https://doi.org/10.1007/s00198-021-05877-8 .
Article7. Papaioannou A, Giangregorio L, Kvern B, Boulos P, Ioannidis G, Adachi JD. The osteoporosis care gap in Canada. BMC Musculoskelet Disord. 2004; 5:11.
Article8. Bell A, Hill MD, Herman RJ, Girard M, Cohen E. Canadian REduction of Atherothrombosis for Continued Health (REACH) Registry Steering Committee. Management of atherothrombotic risk factors in high-risk Canadian outpatients. Can J Cardiol. 2009; 25:345–51.
Article9. Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE Jr, McLellan A, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012; 27:2039–46.
Article10. Mitchell PJ. Best practices in secondary fracture prevention: fracture liaison services. Curr Osteoporos Rep. 2013; 11:52–60.
Article11. Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing. 2016; 45:236–42.
Article12. Metcalfe D, Zogg CK, Judge A, Perry DC, Gabbe B, Willett K, et al. Pay for performance and hip fracture outcomes: an interrupted time series and difference-in-differences analysis in England and Scotland. Bone Joint J. 2019; 101-B:1015–23.13. Gonzalez-Quevedo D, Bautista-Enrique D, Perez-Del-Rio V, Bravo-Bardaji M, Garcia-de-Quevedo D, Tamimi I. Fracture liaison service and mortality in elderly hip fracture patients: a prospective cohort study. Osteoporos Int. 2020; 31:77–84.
Article14. Binkley N, Blank RD, Leslie WD, Lewiecki EM, Eisman JA, Bilezikian JP. Osteoporosis in crisis: it’s time to focus on fracture. J Bone Miner Res. 2017; 32:1391–4.
Article15. Leslie WD, Lix LM. Comparison between various fracture risk assessment tools. Osteoporos Int. 2014; 25:1–21.
Article16. Marques A, Ferreira RJ, Santos E, Loza E, Carmona L, da Silva JA. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis. 2015; 74:1958–67.
Article17. Burden AM, Tanaka Y, Xu L, Ha YC, McCloskey E, Cummings SR, et al. Osteoporosis case ascertainment strategies in European and Asian countries: a comparative review. Osteoporos Int. 2021; 32:817–29.
Article18. McCloskey E, Kanis JA, Johansson H, Harvey N, Oden A, Cooper A, et al. FRAX-based assessment and intervention thresholds: an exploration of thresholds in women aged 50 years and older in the UK. Osteoporos Int. 2015; 26:2091–9.
Article19. Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017; 12:43.
Article20. Giangregorio LM, Leslie WD. Manitoba Bone Density Program. Time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res. 2010; 25:1400–5.
Article21. Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, et al. Imminent risk of fracture after fracture. Osteoporos Int. 2017; 28:775–80.
Article22. Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011; 22:2395–411.
Article23. Beaudoin C, Jean S, Moore L, Gamache P, Bessette L, Ste-Marie LG, et al. Number, location, and time since prior fracture as predictors of future fracture in the elderly from the general population. J Bone Miner Res. 2018; 33:1956–66.
Article24. Kanis JA, Johansson H, Harvey NC, Gudnason V, Sigurdsson G, Siggeirsdottir K, et al. Adjusting conventional FRAX estimates of fracture probability according to the recency of sentinel fractures. Osteoporos Int. 2020; 31:1817–28.
Article25. Kanis JA, Johansson H, Harvey NC, Gudnason V, Sigurdsson G, Siggeirsdottir K, et al. The use of 2-, 5-, and 10-year probabilities to characterize fracture risk after a recent sentinel fracture. Osteoporos Int. 2021; 32:47–54.
Article26. Kanis JA, Johansson H, Harvey NC, Lorentzon M, Liu E, Vandenput L, et al. An assessment of intervention thresholds for very high fracture risk applied to the NOGG guidelines: A report for the National Osteoporosis Guideline Group (NOGG). Osteoporos Int. 2021. Apr. 4. [Epub]. https://doi.org/10.1007/s00198-021-05942-2 .
Article27. Kim HY, Jang EJ, Park B, Kim TY, Shin SA, Ha YC, et al. Development of a Korean Fracture Risk Score (KFRS) for predicting osteoporotic fracture risk: analysis of data from the Korean National Health Insurance Service. PLoS One. 2016; 11:e0158918.
Article28. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020; 105:dgaa048.
Article29. Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018; 391:230–40.
Article30. Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017; 377:1417–27.
Article31. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017; 32:198–202.
Article32. McClung MR. Role of bone-forming agents in the management of osteoporosis. Aging Clin Exp Res. 2021; 33:775–91.
Article33. Brown JP. Antiresorptives: safety concerns-clinical perspective. Toxicol Pathol. 2017; 45:859–63.
Article34. Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015; 30:3–23.
Article35. Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010; 25:2267–94.
Article36. Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014; 29:1–23.
Article37. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 Years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017; 5:513–23.
Article38. Watts NB, Grbic JT, Binkley N, Papapoulos S, Butler PW, Yin X, et al. Invasive oral procedures and events in postmenopausal women with osteoporosis treated with denosumab for up to 10 years. J Clin Endocrinol Metab. 2019; 104:2443–52.
Article39. Ng AC, Png MA, Mohan PC, Koh JS, Howe TS. Atypical femoral fractures: transverse morphology at lateral cortex is a critical feature. J Bone Miner Res. 2014; 29:639–43.
Article40. Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016; 101:3163–70.
Article41. Ferrari S, Libanati C, Lin CJF, Brown JP, Cosman F, Czerwinski E, et al. Relationship between bone mineral density t-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019; 34:1033–40.
Article42. Cummings SR, Cosman F, Lewiecki EM, Schousboe JT, Bauer DC, Black DM, et al. Goal-directed treatment for osteoporosis: a progress report from the ASBMR-NOF working group on goal-directed treatment for osteoporosis. J Bone Miner Res. 2017; 32:3–10.
Article43. Lewiecki EM. Osteoporosis: treat-to-target. Curr Osteoporos Rep. 2017; 15:103–9.
Article44. Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019; 34:632–42.
Article45. Dennison EM, Cooper C, Kanis JA, Bruyere O, Silverman S, McCloskey E, et al. Fracture risk following intermission of osteoporosis therapy. Osteoporos Int. 2019; 30:1733–43.
Article46. Eastell R, Hannon RA, Wenderoth D, Rodriguez-Moreno J, Sawicki A. Effect of stopping risedronate after long-term treatment on bone turnover. J Clin Endocrinol Metab. 2011; 96:3367–73.
Article47. Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–38.
Article48. Brown JP, Morin S, Leslie W, Papaioannou A, Cheung AM, Davison KS, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014; 60:324–33.49. Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012; 27:243–54.
Article50. Black DM, Reid IR, Cauley JA, Cosman F, Leung PC, Lakatos P, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015; 30:934–44.
Article51. Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007; 357:1799–809.
Article52. Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016; 31:16–35.
Article53. Miller PD, Pannacciulli N, Malouf-Sierra J, Singer A, Czerwinski E, Bone HG, et al. Efficacy and safety of denosumab vs. bisphosphonates in postmenopausal women previously treated with oral bisphosphonates. Osteoporos Int. 2020; 31:181–91.
Article54. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019; 104:1595–622.
Article55. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011; 96:972–80.
Article56. Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012; 23:317–26.
Article57. Solling AS, Harslof T, Langdahl B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J Bone Miner Res. 2020; 35:1858–70.
Article58. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018; 33:190–8.
Article59. Ferrari S, Butler PW, Kendler DL, Miller PD, Roux C, Wang AT, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019; 104:3450–61.
Article