Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea
- Affiliations
-
- 1Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Clinical Research Center, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Office for Infection Control, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2515806
- DOI: http://doi.org/10.3346/jkms.2021.36.e115
Abstract
- Background
We performed a prospective survey on the adverse reactions following the first dose of two types of vaccines against coronavirus disease 2019 (COVID-19) in healthcare workers (HCWs) in South Korea.
Methods
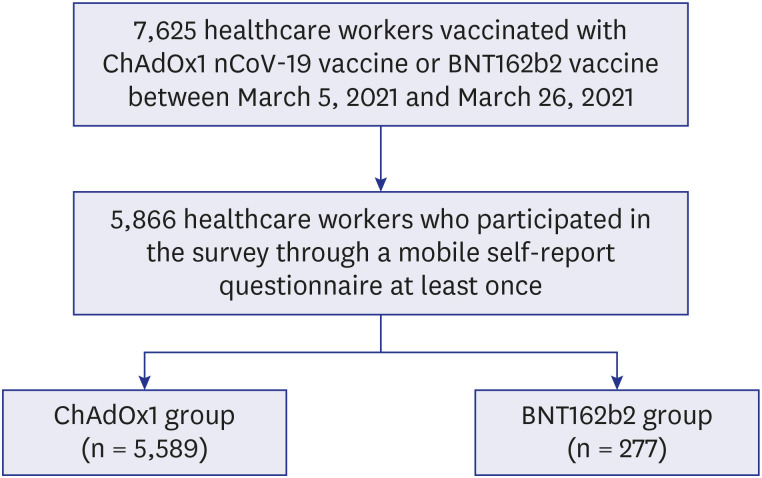
HCWs at a tertiary referral hospital in Seoul, South Korea, received a chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19) or an mRNA-based vaccine (BNT162b2) between March 5 and March 26, 2021. The HCWs were asked to report adverse reactions through a mobile self-report questionnaire for three days after vaccination.
Results
A total of 7,625 HCWs received the first dose of ChAdOx1 or BNT162b2 vaccine during the study period. Of them, 5,866 (76.9%) HCWs (ChAdOx1, n = 5,589 [95.3%]; BNT162b2, n = 277 [4.7%]) participated at least once in the survey, of whom 77% were female and 86% were younger than 50 years. The overall adverse reaction rate was 93% in the ChAdOx1 group and 80% in the BNT162b2 group (P < 0.001). Both local and systemic reactions were more commonly reported in the ChAdOx1 group, and the difference was larger in systemic reactions such as fever and fatigue. In the ChAdOx1 group, the incidence of adverse reactions was significantly higher in females and those in the younger age groups, while the BNT162b2 group showed such difference according to age.
Conclusion
In our prospective survey, vaccine-associated adverse reactions were more commonly reported in the ChAdOx1 group than in the BNT162b2 group. Females and younger age groups experienced vaccine-associated adverse reactions more frequently.
Keyword
Figure
Cited by 5 articles
-
Adverse Reactions of the Second Dose of the BNT162b2 mRNA COVID-19 Vaccine in Healthcare Workers in Korea
Yun Woo Lee, So Yun Lim, Ji-Hyang Lee, Joon Seo Lim, Miseo Kim, Seonhee Kwon, Jiyeon Joo, Sun Hee Kwak, Eun Ok Kim, Jiwon Jung, Hyouk-Soo Kwon, Tae-Bum Kim, Sung-Han Kim, Seongman Bae
J Korean Med Sci. 2021;36(21):e153. doi: 10.3346/jkms.2021.36.e153.Emergency Department Utilization by In-hospital Healthcare Workers after COVID-19 Vaccination
Min Ji Park, Yoo Jin Choi, Sangchun Choi
J Korean Med Sci. 2021;36(27):e196. doi: 10.3346/jkms.2021.36.e196.Safety Monitoring after the BNT162b2 COVID-19 Vaccine among Adults Aged 75 Years or Older
Youn Young Choi, Min-Kyung Kim, Hyeok Choon Kwon, Gunn Hee Kim
J Korean Med Sci. 2021;36(45):e318. doi: 10.3346/jkms.2021.36.e318.Rhinovirus Incidence Rates Indicate We Are Tired of Non-pharmacological Interventions Against Coronavirus Disease 2019
Min-Chul Kim, Joung Ha Park, Seong-Ho Choi, Jin-Won Chung
J Korean Med Sci. 2021;37(2):e15. doi: 10.3346/jkms.2022.37.e15.Clinical Features of Patients Presenting to the Emergency Department With Cardiovascular Adverse Reactions After COVID-19 mRNA Vaccination
Tae Hoon Oh, Seon Hee Woo, Sungyoup Hong, Carol Lee, Woon Jeong Lee, Si Kyoung Jeong
J Korean Med Sci. 2022;37(9):e73. doi: 10.3346/jkms.2022.37.e73.
Reference
-
1. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.
Article2. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID: 33306989.3. Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021; 396(10267):1979–1993. PMID: 33220855.4. Centers for Disease Control and Prevention. Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Updated December 13, 2020. Accessed March 28, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.5. Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020; 383(25):2439–2450. PMID: 33053279.
Article6. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021; 21(4):195–197. PMID: 33674759.
Article7. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.
Article8. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020; 396(10249):467–478. PMID: 32702298.9. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021; 325(8):780–781. PMID: 33475702.
Article10. Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine. 2013; 31(51):6107–6112. PMID: 24120547.
Article11. Klein SL, Roberts C. Sex Hormones and Immunity to Infection. Berlin, Germany: Springer Berlin;2014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Events in Healthcare Workers after the First Dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 Vaccination: a Single Center Experience
- Comparison of Antibody and T Cell Responses Induced by Single Doses of ChAdOx1 nCoV-19 and BNT162b2 Vaccines
- Comparison of antibody responses after the 1st and 2nd doses of COVID-19 vaccine with those of patients with mild or severe COVID-19
- Adverse Reactions after the Second Dose of ChAdOx1 COVID-19 Vaccine in H ealthcare Workers
- Livedo reticularis following administration of ChAdOx1 nCoV-19 vaccine (AZD1222): a report of two cases