Intest Res.
2021 Apr;19(2):194-205. 10.5217/ir.2020.00021.
Yarrow oil ameliorates ulcerative colitis in mice model via regulating the NF-κB and PPAR-γ pathways
- Affiliations
-
- 1Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, Al-Ahsa, Kingdom of Saudi Arabia
- 2Department of Pharmacognosy, College of Pharmacy, University of Zagazig, Zagazig, Egypt
- 3Department of Rheumatology and Rehabilitation, Faculty of Medicine, Sohag University, Sohag, Egypt
- 4Department of Biochemistry, Faculty of Pharmacy, Al-Azhar University, Assiut, Egypt
- 5Department of Histology and Cell Biology, Faculty of Medicine, Assiut University, Assiut, Egypt
- KMID: 2515478
- DOI: http://doi.org/10.5217/ir.2020.00021
Abstract
- Background/Aims
Ulcerative colitis (UC) is a chronic inflammatory disorder with indefinite etiology; however, environmental, genetic, immune factors and microbial agents could be implicated in its pathogenesis. UC treatment is lifelong, therefore; the potential side effects and cost of the therapy are significant. Yarrow is a promising medicinal plant with the ability to treat many disorders, owing to its bioactive compounds especially the essential oil. The main aim of this research was to investigate the therapeutic effect of the yarrow oil on colitis including the involved mechanism of action.
Methods
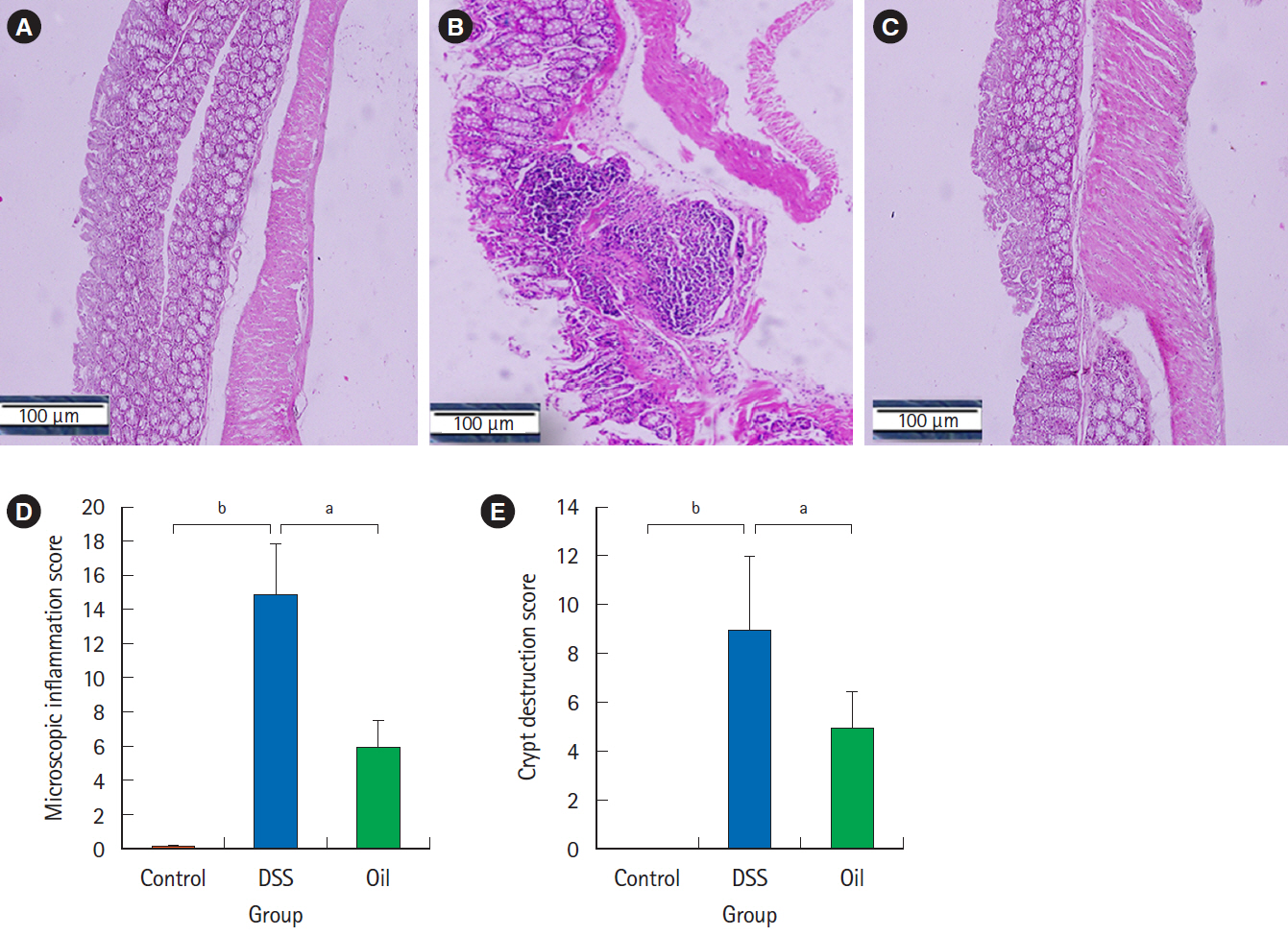
In 21-female C57BL/6 mice were divided into 3 groups; control group, colitis model group, and oil-treated group. Groups 2 and 3 received 5% dextran sulfate sodium (DSS) in drinking water for 9 days, and concomitantly, only group 3 was given 100 mg/kg yarrow oil. Mice were examined for their body weight, stool consistency and bleeding, and the disease activity indexes were calculated.
Results
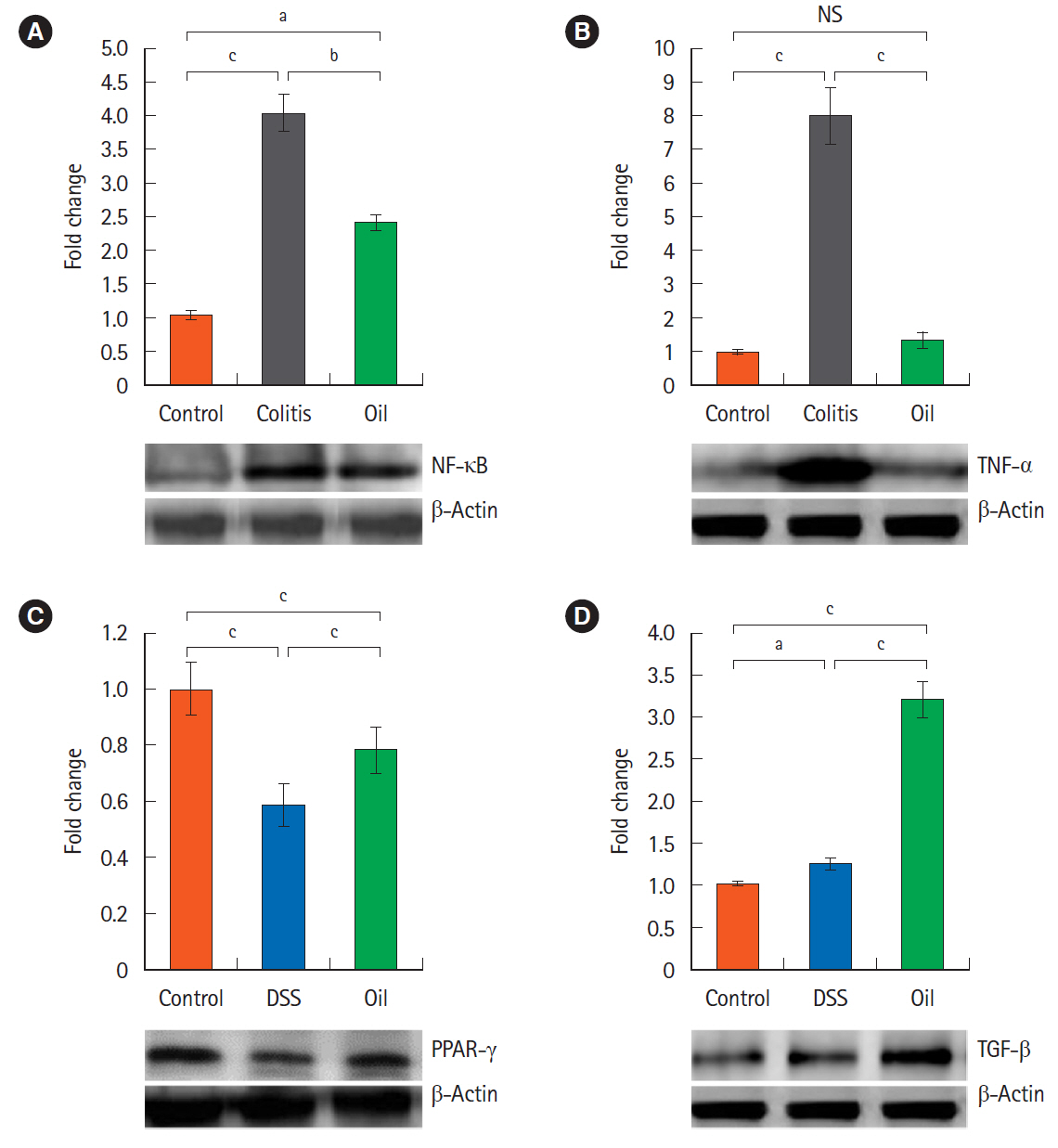
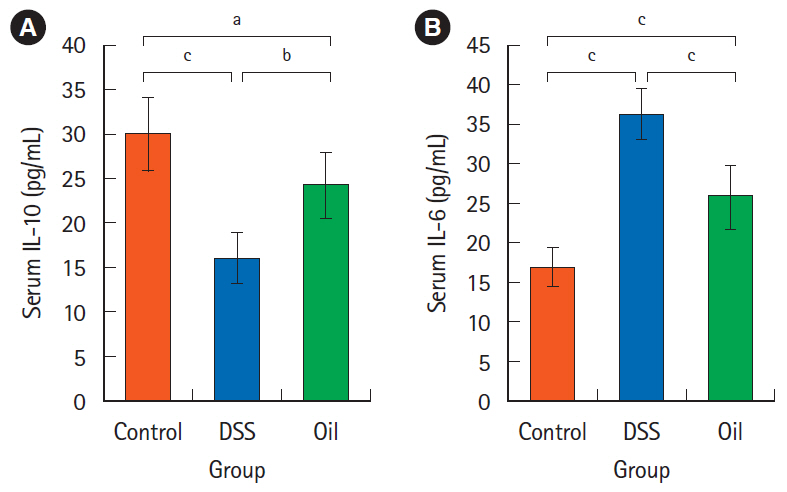
Oral administration of yarrow oil markedly repressed the severity of UC via the reduction of the inflammatory signs and restoring colon length. The oil was able to down-regulate nuclear factor kappa light chain enhancer of activated B cells (NF-κB), up-regulate peroxisome proliferator-activated receptor gamma (PPAR-γ), and enhance transforming growth factor-β expression. The oil normalized the tumor necrosis factor-α expression, restored the normal serum level of interleukin-10 (IL-10) and reduced the serum level of IL-6.
Conclusions
Yarrow oil mitigated UC symptoms and regulated the inflammatory cytokines secretion via regulation of NF-κB and PPAR-γ pathways in the mice model, however, this recommendation requires further investigations using clinical studies to confirm the use of the oil on humans.
Figure
Reference
-
1. Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013; 58:519–525.
Article2. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016; 13:13–27.
Article3. Gordillo J, Zabana Y, Garcia-Planella E, et al. Prevalence and risk factors for colorectal adenomas in patients with ulcerative colitis. United European Gastroenterol J. 2018; 6:322–330.
Article4. Kim Y, Wu AG, Jaja-Chimedza A, et al. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One. 2017; 12:e0184709.
Article5. Tripathi K, Feuerstein JD. New developments in ulcerative colitis: latest evidence on management, treatment, and maintenance. Drugs Context. 2019; 8:212572.
Article6. Shah R, Peethambaran B. Anti-inflammatory and anti-microbial properties of Achillea millefolium in acne treatment. In : Chatterjee S, Jungraithmayr W, Bagchi D, editors. Immunity and inflammation in health and disease: emerging roles of nutraceuticals and functional foods in immune support. London: Academic Press;2018. p. 241–248.7. Baretta IP, Felizardo RA, Bimbato VF, et al. Anxiolytic-like effects of acute and chronic treatment with Achillea millefolium L. extract. J Ethnopharmacol. 2012; 140:46–54.
Article8. Miller FM, Chow LM. Alkaloids of Achillea millefolium L. I. Isolation and characterization of Achilleine1,2. J Am Chem Soc. 1954; 76:1353–1354.
Article9. de Souza P, Gasparotto A Jr, Crestani S, et al. Hypotensive mechanism of the extracts and artemetin isolated from Achillea millefolium L. (Asteraceae) in rats. Phytomedicine. 2011; 18:819–825.
Article10. Verma RS, Joshi N, Padalia RC, et al. Chemical composition and allelopathic, antibacterial, antifungal and in vitro acetylcholinesterase inhibitory activities of yarrow (Achillea millefolium L.) native to India. Ind Crops Prod. 2017; 104:144–155.
Article11. Marzouki H, Piras A, Porcedda S, Falconieri D, Bagdonaite E. Influence of extraction methods on the composition of essential oils of Achillea millefolium L. from Lithuania. J Biodivers Manage Forestry. 2014; 3:1000133.
Article12. Nadim MM, Malik AA, Ahmad J, Bakshi SK. The essential oil composition of Achillea millefolium L. cultivated under tropical condition in India. World J Agric Sci. 2011; 7:561–565.13. Tadić V, Arsić I, Zvezdanović J, Zugić A, Cvetković D, Pavkov S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J Ethnopharmacol. 2017; 199:138–148.
Article14. Aydin S, Sevindik E. Achillea millefolium L. subsp. millefolium essential oil’s antifungal effect. Eur J Biol Res. 2018; 8:153–156.15. Applequist WL, Moerman DE. Yarrow (Achillea millefolium L.): a neglected panacea? A review of ethnobotany, bioactivity, and biomedical research. Econ Bot. 2011; 65:209.
Article16. Ali SI, Gopalakrishnan B, Venkatesalu V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: a review. Phytother Res. 2017; 31:1140–1161.
Article17. Karakaya S, El SN, Karagozlu N, Sahin S, Sumnu G, Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J Food Sci Technol. 2014; 51:1056–1065.
Article18. Dong S, Chen M, Dai F, et al. 5-Hydroxytryptamine (5-HT)-exacerbated DSS-induced colitis is associated with elevated NADPH oxidase expression in the colon. J Cell Biochem. 2019; 120:9230–9242.
Article19. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014; 104:15.25.1–15.25.14.
Article20. Wanes D, Jabri MA, Tounsi H, et al. Chemical characterization of bioactive components of Rosa canina extract and its protective effect on dextran sulfate sodium-induced intestinal bowel disease in a mouse model. J Med Food. 2020; 23:1109–1119.
Article21. Alzahrani AM, Hanieh H, Ibrahim HM, et al. Enhancing miR132 expression by aryl hydrocarbon receptor attenuates tumorigenesis associated with chronic colitis. Int Immunopharmacol. 2017; 52:342–351.
Article22. Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993; 38:1722–1734.
Article23. Zhang Z, Wu X, Cao S, et al. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget. 2016; 7:31790–31799.
Article24. Zuo L, Huang Z, Dong L, et al. Targeting delivery of anti-TNFalpha oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut. 2010; 59:470–479.
Article25. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007; 2:541–546.
Article26. Almeida JR, Souza GR, Silva JC, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. ScientificWorldJournal. 2013; 2013:808460.
Article27. Ihara S, Hirata Y, Koike K. TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol. 2017; 52:777–787.
Article28. Fonseca-Camarillo G, Yamamoto-Furusho JK. Immunoregulatory pathways involved in inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:2188–2193.
Article29. Katsanos KH, Papadakis KA. Inflammatory bowel disease: updates on molecular targets for biologics. Gut Liver. 2017; 11:455–463.
Article30. Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol. 2018; 30:1–10.
Article31. MacFarlane EG, Haupt J, Dietz HC, Shore EM. TGF-β family signaling in connective tissue and skeletal diseases. Cold Spring Harb Perspect Biol. 2017; 9:a022269.
Article32. Moses HL, Roberts AB, Derynck R. The discovery and early days of TGF-β: a historical perspective. Cold Spring Harb Perspect Biol. 2016; 8:a021865.
Article33. Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013; 19:5633–5644.
Article34. Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998; 22:382–389.
Article35. Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017; 9:a022236.
Article36. Geginat J, Paroni M, Kastirr I, Larghi P, Pagani M, Abrignani S. Reverse plasticity: TGF-β and IL-6 induce Th1-to-Th17-cell transdifferentiation in the gut. Eur J Immunol. 2016; 46:2306–2310.
Article37. Jones LL, Alli R, Li B, Geiger TL. Differential t cell cytokine receptivity and not signal quality distinguishes IL-6 and IL-10 signaling during Th17 differentiation. J Immunol. 2016; 196:2973–2985.
Article38. Li J, Zhong W, Wang W, et al. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PLoS One. 2014; 9:e87810.
Article39. Dubuquoy L, Jansson EA, Deeb S, et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003; 124:1265–1276.
Article40. Annese V, Rogai F, Settesoldi A, Bagnoli S. PPARγ in inflammatory bowel disease. PPAR Res. 2012; 2012:620839.41. Yamamoto-Furusho JK, Peñaloza-Coronel A, Sánchez-Muñoz F, Barreto-Zuñiga R, Dominguez-Lopez A. Peroxisome proliferator-activated receptor-gamma (PPAR-γ) expression is downregulated in patients with active ulcerative colitis. Inflamm Bowel Dis. 2011; 17:680–681.
Article42. Martin H. Role of PPAR-gamma in inflammation: prospects for therapeutic intervention by food components. Mutat Res. 2010; 690:57–63.
Article43. Ramadan M, Goeters S, Watzer B, et al. Chamazulene carboxylic acid and matricin: a natural profen and its natural prodrug, identified through similarity to synthetic drug substances. J Nat Prod. 2006; 69:1041–1045.
Article44. Tambe Y, Tsujiuchi H, Honda G, Ikeshiro Y, Tanaka S. Gastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, beta-caryophyllene. Planta Med. 1996; 62:469–470.
Article45. Younis NS, Mohamed ME. β-Caryophyllene as a potential protective agent against myocardial injury: the role of toll-like receptors. Molecules. 2019; 24:1929.
Article46. Sitarek P, Rijo P, Garcia C, et al. Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid Med Cell Longev. 2017; 2017:7384061.47. Querio G, Antoniotti S, Foglietta F, et al. Chamazulene attenuates ROS levels in bovine aortic endothelial cells exposed to high glucose concentrations and hydrogen peroxide. Front Physiol. 2018; 9:246.
Article48. Santos FA, Silva RM, Campos AR, De Araújo RP, Lima Júnior RC, Rao VS. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem Toxicol. 2004; 42:579–584.
Article49. Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003; 97:250–256.
Article50. Valente J, Zuzarte M, Gonçalves MJ, et al. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem Toxicol. 2013; 62:349–354.
Article51. Zou L, Zhang Y, Li W, et al. Comparison of chemical profiles, anti-inflammatory activity, and UPLC-Q-TOF/MS-based metabolomics in endotoxic fever rats between synthetic borneol and natural borneol. Molecules. 2017; 22:1446.
Article52. Zhang X, Xu F, Liu L, et al. (+)-Borneol improves the efficacy of edaravone against DSS-induced colitis by promoting M2 macrophages polarization via JAK2-STAT3 signaling pathway. Int Immunopharmacol. 2017; 53:1–10.
Article53. Fernandes ES, Passos GF, Medeiros R, et al. Anti-inflammatory effects of compounds alpha-humulene and (-)-transcaryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol. 2007; 569:228–236.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Long Noncoding RNA SNHG15 Ameliorates Hypoxia/Ischemia-Induced Neuronal Damage by Regulating miR-302a-3p/STAT1/NF-κB Axis
- Paper “Inhibition of Long Noncoding RNA SNHG15 Ameliorates Hypoxia/Ischemia-Induced Neuronal Damage by Regulating miR-302a-3p/STAT1/NF-κB Axis” by Hu C, et al.[Yonsei Med J 2021;62(4):325-337]
- Lovastatin derivative dehydrolovastatin ameliorates ulcerative colitis in mice by suppressing NF-κB and inflammatory cytokine expression
- Hemin attenuates bleomycin-induced lung fibrosis in mice by regulating the TGF-ββ1/MAPK and AMPK/SIRT1/PGC-1αα/HO-1/ NF-κκB pathways
- The effect of Hericium erinaceum on the prevention of chemically induced experimental colitis in rats