Intest Res.
2021 Apr;19(2):158-170. 10.5217/ir.2020.00008.
The impact of tobacco smoking on treatment choice and efficacy in inflammatory bowel disease
- Affiliations
-
- 1Department of Gastroenterology, Eastern Health, Box Hill Hospital, Box Hill, Australia
- KMID: 2515475
- DOI: http://doi.org/10.5217/ir.2020.00008
Abstract
- Smoking significantly increases the risk of developing and worsens Crohn’s disease (CD), yet protects against the development and reduces the severity of ulcerative colitis. It is less clear whether smoking impacts the efficacy of therapeutics in inflammatory bowel disease (IBD). We review the literature regarding the relationship between smoking and the efficacy of medical and surgical therapy in IBD. Smoking is associated with alterations in thiopurine metabolism and may affect time to disease relapse. The outcomes of anti-tumor necrosis factor therapy in active smokers appear neutral with data lacking for newer biologics. Smoking increases the risk of postoperative recurrence in those requiring resection for CD, likely attributable to perturbations of the gut microbiota although further implications of these for disease onset/progression and treatment efficacy remain unclear. Multiple lifestyle and psychosocial confounders are likely under-recognized cofactors in the association between smoking and IBD. Despite the widely promulgated risks associated with cigarette smoking in CD, more incisive data are required to further elucidate the actual relationship between smoking and disease pathways, while accounting for the several negative cofactors prevalent in smokers which cast uncertainty on the magnitude of the direct effect of smoking on disease pathophysiology and the efficacy of therapy.
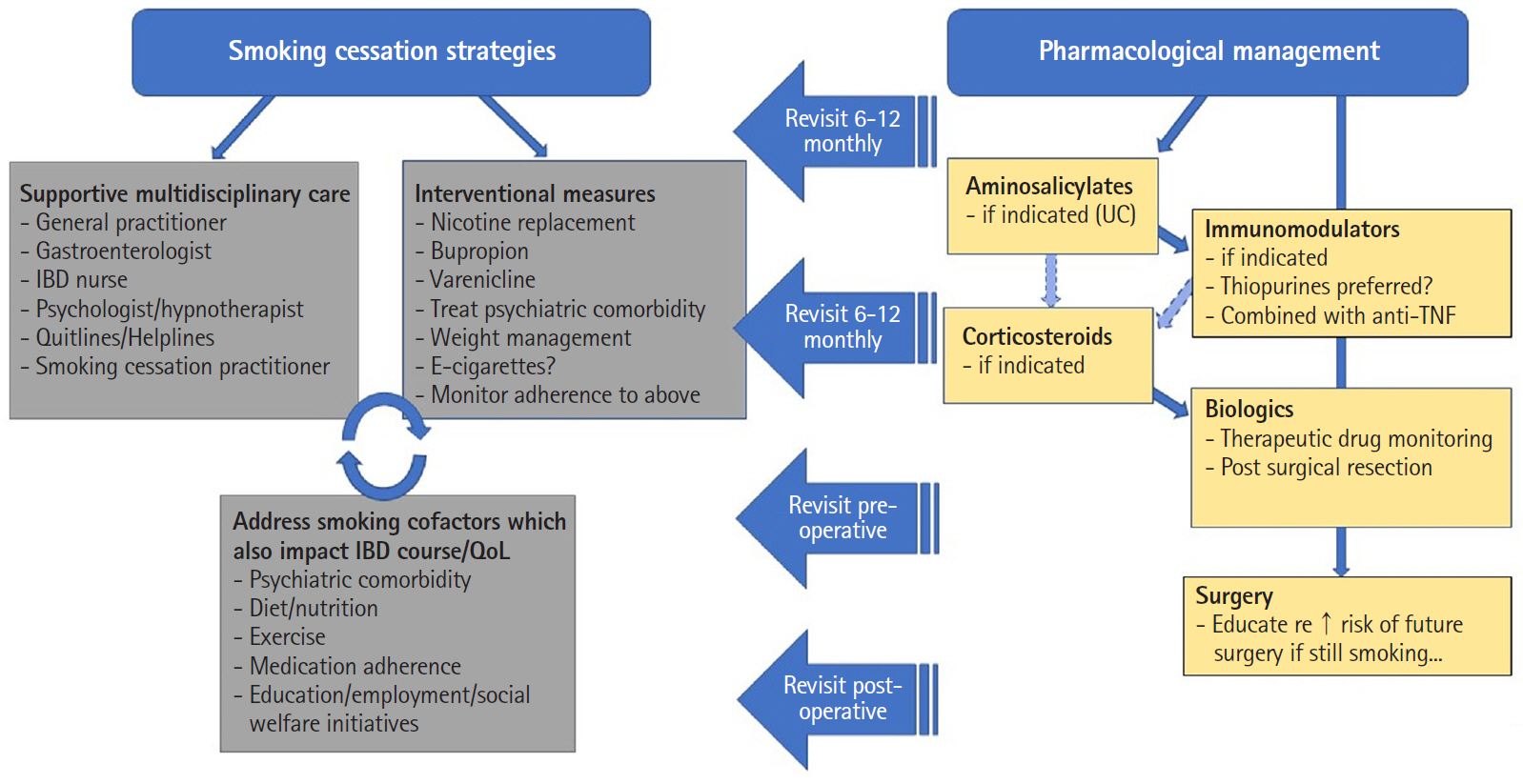
Figure
Reference
-
1. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking–50 years of progress: a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention (US);2014.2. Australian Institute of Health and Welfare (AIHW). Australia’s health 2018. Australia’s health series no. 16. AUS 221. Canberra: AIHW;2018.3. Thomas T, Chandan JS, Li VSW, et al. Global smoking trends in inflammatory bowel disease: a systematic review of inception cohorts. PLoS One. 2019; 14:e0221961.
Article4. Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006; 81:1462–1471.
Article5. Boyko EJ, Perera DR, Koepsell TD, Keane EM, Inui TS. Effects of cigarette smoking on the clinical course of ulcerative colitis. Scand J Gastroenterol. 1988; 23:1147–1152.
Article6. Meijer B, Seinen ML, Hosman T, et al. High inter-individual variability of serum xanthine oxidoreductase activity in IBD patients. Nucleosides Nucleotides Nucleic Acids. 2018; 37:317–323.
Article7. Poon SS, Asher R, Jackson R, et al. Body mass index and smoking affect thioguanine nucleotide levels in inflammatory bowel disease. J Crohns Colitis. 2015; 9:640–646.
Article8. Kayyali US, Budhiraja R, Pennella CM, et al. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol Appl Pharmacol. 2003; 188:59–68.
Article9. Domènech E, Carrión S, Garcia-Planella E, et al. Smoking status and response to thiopurines in steroid-dependent inflammatory bowel disease. Inflamm Bowel Dis. 2011; 17:971–975.
Article10. Mowat C, Arnott I, Cahill A, et al. Mercaptopurine versus placebo to prevent recurrence of Crohn’s disease after surgical resection (TOPPIC): a multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2016; 1:273–282.11. Lakatos PL, Szamosi T, Czegledi Z, et al. S1143 Azathioprine/biological therapy does prevent surgery but not reoperation in smokers with Crohn’s disease. Gastroenterol. 2009; 136:A199.
Article12. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015; 385:1406–1417.
Article13. Sokol H, Seksik P, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Current smoking, not duration of remission, delays Crohn’s disease relapse following azathioprine withdrawal. Inflamm Bowel Dis. 2010; 16:362–363.
Article14. Treton X, Bouhnik Y, Mary JY, et al. Azathioprine withdrawal in patients with Crohn’s disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. 2009; 7:80–85.
Article15. Chauvin A, Le Thuaut A, Belhassan M, et al. Infliximab as a bridge to remission maintained by antimetabolite therapy in Crohn’s disease: a retrospective study. Dig Liver Dis. 2014; 46:695–700.
Article16. Saevarsdottir S, Wedrén S, Seddighzadeh M, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum. 2011; 63:26–36.
Article17. Teitsma XM, Jacobs JWG, Welsing PMJ, et al. Inadequate response to treat-to-target methotrexate therapy in patients with new-onset rheumatoid arthritis: development and validation of clinical predictors. Ann Rheum Dis. 2018; 77:1261–1267.
Article18. Parsi MA, Achkar JP, Richardson S, et al. Predictors of response to infliximab in patients with Crohn’s disease. Gastroenterology. 2002; 123:707–713.
Article19. Arnott ID, McNeill G, Satsangi J. An analysis of factors influencing short-term and sustained response to infliximab treatment for Crohn’s disease. Aliment Pharmacol Ther. 2003; 17:1451–1457.
Article20. Kong JY, Bundell CS, Pawlik J, Hollingsworth PN, Forbes GM. Smoking is associated with low trough serum infliximab levels and presence of anti-infliximab antibody in maintenance treatment of inflammatory bowel disease (IBD). J Gastroenterol Hepatol. 2011; 26:56–67.21. Glossop JR, Dawes PT, Mattey DL. Association between cigarette smoking and release of tumour necrosis factor alpha and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology (Oxford). 2006; 45:1223–1229.
Article22. Narula N, Fedorak RN. Does smoking reduce infliximab’s effectiveness against Crohn’s disease? Can J Gastroenterol. 2009; 23:121–125.
Article23. Inamdar S, Volfson A, Rosen L, Sunday S, Katz S, Sultan K. Smoking and early infliximab response in Crohn’s disease: a meta-analysis. J Crohns Colitis. 2015; 9:140–146.
Article24. Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY Consortium. Am J Gastroenterol. 2016; 111:1147–1155.
Article25. Petersen ER, Søndergaard HB, Laursen JH, et al. Smoking is associated with increased disease activity during natalizumab treatment in multiple sclerosis. Mult Scler. 2019; 25:1298–1305.
Article26. Hedström AK, Ryner M, Fink K, et al. Smoking and risk of treatment-induced neutralizing antibodies to interferon β-1a. Mult Scler. 2014; 20:445–450.
Article27. Anzengruber F, Augustin M, Radtke MA, et al. Smoking does not alter the therapy response to systemic anti-psoriatic therapies: a two-country, multi-centre, prospective, non-interventional study. Acta Derm Venereol. 2019; 99:871–877.
Article28. Umezawa Y, Saeki H, Nakagawa H. Some clinical factors affecting quality of the response to ustekinumab for psoriasis. J Dermatol. 2014; 41:690–696.
Article29. Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibodyinduced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014; 63:567–577.
Article30. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article31. Karataş A, Öz B, Dalkiliç E, et al. Cigarette smoking does not affect treatment response to tofacitinib in rheumatoid arthritis. Arthritis Rheumatol. 2018; 70(Suppl 10):1554.32. Pfizer. Pfizer announces modification to ongoing tofacitinib FDA post-marketing requirement study in patients with rheumatoid arthritis [Internet]. c2019 [cited 2020 Jan 6]. https://investors.pfizer.com/investor-news/press-release-details/2019/Pfizer-Announces-Modification-to-Ongoing-Tofacitnib-FDA-Post-Marketing-Requirement-Study-in-Patients-with-Rheumatoid-Arthritis/default.aspx.33. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019; 50:1068–1076.
Article34. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018; 24:2258–2265.
Article35. Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018; 24:5–14.
Article36. Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017; 11:1180–1199.
Article37. Sood A, Mahajan R, Singh A, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. 2019; 13:1311–1317.
Article38. Lee SH, Yun Y, Kim SJ, et al. Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study. J Clin Med. 2018; 7:282.
Article39. Biedermann L, Brülisauer K, Zeitz J, et al. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis. 2014; 20:1496–1501.40. Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005; 54:237–241.
Article41. Auzolle C, Nancey S, Tran-Minh ML, et al. Male gender, active smoking and previous intestinal resection are risk factors for post-operative endoscopic recurrence in Crohn’s disease: results from a prospective cohort study. Aliment Pharmacol Ther. 2018; 48:924–932.
Article42. Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008; 23:1213–1221.
Article43. Kuenzig ME, Lee SM, Eksteen B, et al. Smoking influences the need for surgery in patients with the inflammatory bowel diseases: a systematic review and meta-analysis incorporating disease duration. BMC Gastroenterol. 2016; 16:143.
Article44. Gustavsson A, Magnuson A, Blomberg B, Andersson M, Halfvarson J, Tysk C. Smoking is a risk factor for recurrence of intestinal stricture after endoscopic dilation in Crohn’s disease. Aliment Pharmacol Ther. 2013; 37:430–437.
Article45. Di Stefano A, Capelli A, Lusuardi M, et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998; 158:1277–1285.
Article46. MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005; 2:258–266.
Article47. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004; 43:1731–1737.48. Berkowitz L, Schultz BM, Salazar GA, et al. Impact of cigarette smoking on the gastrointestinal tract inflammation: opposing effects in Crohn’s disease and ulcerative colitis. Front Immunol. 2018; 9:74.
Article49. Li LF, Chan RL, Lu L, et al. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review). Int J Mol Med. 2014; 34:372–380.
Article50. Ueno A, Jijon H, Traves S, et al. Opposing effects of smoking in ulcerative colitis and Crohn’s disease may be explained by differential effects on dendritic cells. Inflamm Bowel Dis. 2014; 20:800–810.
Article51. Mackern-Oberti JP, Riquelme SA, Llanos C, et al. Heme oxygenase-1 as a target for the design of gene and pharmaceutical therapies for autoimmune diseases. Curr Gene Ther. 2014; 14:218–235.
Article52. Sher ME, Bank S, Greenberg R, et al. The influence of cigarette smoking on cytokine levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 1999; 5:73–78.
Article53. Lee G, Jung KH, Shin D, et al. Cigarette smoking triggers colitis by IFN-γ+CD4+T cells. Front Immunol. 2017; 8:1344.54. Getliffe KM, Al Dulaimi D, Martin-Ruiz C, et al. Lymphocyte telomere dynamics and telomerase activity in inflammatory bowel disease: effect of drugs and smoking. Aliment Pharmacol Ther. 2005; 21:121–131.
Article55. Bridger S, Lee JC, Bjarnason I, Jones JE, Macpherson AJ. In siblings with similar genetic susceptibility for inflammatory bowel disease, smokers tend to develop Crohn’s disease and non-smokers develop ulcerative colitis. Gut. 2002; 51:21–25.
Article56. Nielsen OH, Bjerrum JT, Csillag C, Nielsen FC, Olsen J. Influence of smoking on colonic gene expression profile in Crohn’s disease. PLoS One. 2009; 4:e6210.
Article57. Opstelten JL, Plassais J, van Mil SW, et al. Gut microbial diversity is reduced in smokers with Crohn’s disease. Inflamm Bowel Dis. 2016; 22:2070–2077.
Article58. Benjamin JL, Hedin CR, Koutsoumpas A, et al. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm Bowel Dis. 2012; 18:1092–1100.
Article59. Kaczynski AT, Manske SR, Mannell RC, Grewal K. Smoking and physical activity: a systematic review. Am J Health Behav. 2008; 32:93–110.
Article60. Engels M, Cross RK, Long MD. Exercise in patients with inflammatory bowel diseases: current perspectives. Clin Exp Gastroenterol. 2017; 11:1–11.
Article61. Jones PD, Kappelman MD, Martin CF, Chen W, Sandler RS, Long MD. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflamm Bowel Dis. 2015; 21:1063–1071.
Article62. Ng V, Millard W, Lebrun C, Howard J. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin J Sport Med. 2007; 17:384–388.
Article63. Loudon CP, Corroll V, Butcher J, Rawsthorne P, Bernstein CN. The effects of physical exercise on patients with Crohn’s disease. Am J Gastroenterol. 1999; 94:697–703.
Article64. Niewiadomski O, Studd C, Wilson J, et al. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern Med J. 2016; 46:669–676.
Article65. Xu L, Lochhead P, Ko Y, Claggett B, Leong RW, Ananthakrishnan AN. Systematic review with meta-analysis: breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2017; 46:780–789.
Article66. MacLean RR, Cowan A, Vernarelli JA. More to gain: dietary energy density is related to smoking status in US adults. BMC Public Health. 2018; 18:365.
Article67. Amre DK, D’Souza S, Morgan K, et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol. 2007; 102:2016–2025.
Article68. Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013; 145:970–977.
Article69. Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019; 157:440–450.
Article70. Muff C, Dragano N, Jöckel KH, et al. Is the co-occurrence of smoking and poor consumption of fruits and vegetables confounded by socioeconomic conditions? Int J Public Health. 2010; 55:339–346.
Article71. Sherman BW, Lynch WD. The association of smoking with medical treatment adherence in the workforce of a large employer. Patient Prefer Adherence. 2014; 8:477–486.
Article72. Lee J, Jee SR, Kim HW, et al. Factors associated with low adherence to oral 5-aminosalicylic acid in patients with ulcerative colitis. PLoS One. 2019; 14:e0214129.
Article73. Lopez A, Billioud V, Peyrin-Biroulet C, Peyrin-Biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013; 19:1528–1533.74. Nielsen MJ, Nørgaard M, Holland-Fisher P, Christensen LA. Self-reported antenatal adherence to medical treatment among pregnant women with Crohn’s disease. Aliment Pharmacol Ther. 2010; 32:49–58.
Article75. Tribbick D, Salzberg M, Ftanou M, et al. Prevalence of mental health disorders in inflammatory bowel disease: an Australian outpatient cohort. Clin Exp Gastroenterol. 2015; 8:197–204.76. Porcelli P, Zaka S, Centonze S, Sisto G. Psychological distress and levels of disease activity in inflammatory bowel disease. Ital J Gastroenterol. 1994; 26:111–115.77. Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004; 66:79–84.
Article78. Mikocka-Walus A, Pittet V, Rossel JB, von Känel R; Swiss IBD Cohort Study Group. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016; 14:829–835.79. Sobell LC, Sobell MB, Agrawal S. Self-change and dual recoveries among individuals with alcohol and tobacco problems: current knowledge and future directions. Alcohol Clin Exp Res. 2002; 26:1936–1938.
Article80. De Leon J, Rendon DM, Baca-Garcia E, et al. Association between smoking and alcohol use in the general population: stable and unstable odds ratios across two years in two different countries. Alcohol Alcohol. 2007; 42:252–257.
Article81. Rajabi A, Dehghani M, Shojaei A, Farjam M, Motevalian SA. Association between tobacco smoking and opioid use: a meta-analysis. Addict Behav. 2019; 92:225–235.
Article82. Persaud R. Smokers’ rights to health care. J Med Ethics. 1995; 21:281–287.
Article83. Siu AL; U.S. Preventive Services Task Force. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015; 163:622–634.
Article84. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults: United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017; 65:1457–1464.85. Biedermann L, Fournier N, Misselwitz B, et al. High rates of smoking especially in female Crohn’s disease patients and low use of supportive measures to achieve smoking cessation: data from the Swiss IBD cohort study. J Crohns Colitis. 2015; 9:819–829.
Article86. Stephens WE. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob Control. 2018; 27:10–17.
Article87. Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016; 9–CD010216.
Article88. McNeill A, Brose LS, Calder R, Bauld L, Robson D. Evidence review of e-cigarettes and heated tobacco products 2018: a report commissioned by Public Health England. London: Public Health England;2018.89. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019; 380:629–637.
Article90. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013; 382:1629–1637.
Article91. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013; 8:e66317.
Article92. Al-Delaimy WK, Myers MG, Leas EC, Strong DR, Hofstetter CR. E-cigarette use in the past and quitting behavior in the future: a population-based study. Am J Public Health. 2015; 105:1213–1219.
Article93. Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med. 2014; 174:812–813.
Article94. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020; 382:1589–1598.
Article95. Katz MG, Russell KW. Injury from e-cigarette explosion. N Engl J Med. 2019; 380:2460.
Article96. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin: final report. N Engl J Med. 2020; 382:903–916.
Article97. Stewart CJ, Auchtung TA, Ajami NJ, et al. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study. PeerJ. 2018; 6:e4693.
Article98. Chong C, Rahman A, Loonat K, Sagar RC, Selinger CP. Current smoking habits in British IBD patients in the age of e-cigarettes. BMJ Open Gastroenterol. 2019; 6:e000309.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Implications of Graphic Cigarette Warning Labels on Smoking Behavior: An International Perspective
- Passive Smoking and Lung Cancer
- The Impact of Heated Tobacco Products on Smoking Cessation, Tobacco Use, and Tobacco Sales in South Korea
- Scientific Evidence for the Addictiveness of Tobacco and Smoking Cessation in Tobacco Litigation
- Nutritional Support in Patients with Inflammatory Bowel Diseases