J Rhinol.
2021 Mar;28(1):1-13. 10.18787/jr.2020.00321.
Clinical Reviews of COVID-19 for Otorhinolaryngologists
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Konyang University College of Medicine, Daejeon, Korea
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Dankook University, College of Medicine, Cheonan, Korea
- KMID: 2513837
- DOI: http://doi.org/10.18787/jr.2020.00321
Abstract
- The novel SARS-CoV-2 virus that causes COVID-19 has emerged rapidly and the virus has caused a global pandemic since it was first diagnosed in December 2019. SARS-CoV-2 is the seventh virus associated with human transmission among corona viruses. Otorhinolaryngologists could be vulnerable to this viral transmission due to the high viral load in the nasal cavity and nasopharynx. Hence, it is essential to understand the novel COVID-19 from the perspective of otorhinolaryngologists. We provide literature reviews of previous human coronaviruses and the novel COVID-19 with clinical hallmarks, diagnostic approaches, and possible treatment options. Further study is necessary to elucidate viral features and standardize treatment protocols with curable anti-viral agents and vaccines.
Keyword
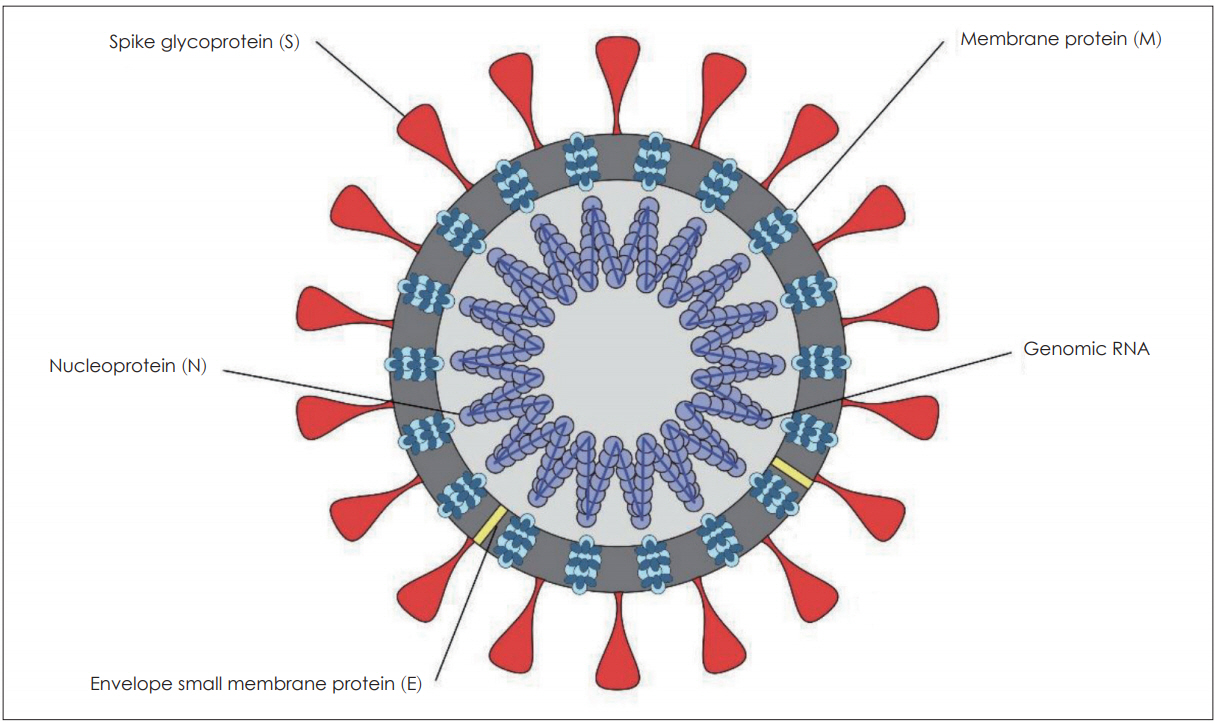
Figure
Cited by 1 articles
-
Clinical Considerations in Otorhinolaryngology Practice in COVID-19 Pandemic Era
Soo Ah Son, Se Hwan Hwang
Korean J Otorhinolaryngol-Head Neck Surg. 2021;64(5):297-303. doi: 10.3342/kjorl-hns.2021.00178.
Reference
-
References
1. Central disease control headquaters. COVID-19 response guidelines, 7th. http://ncov.mohw.go.kr/duBoardList.do?brdId=2&brdGubun=28/2020.2. Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005; 24(11 Suppl):S223–7. discussion S6.3. Falsey AR, Dallal GE, Formica MA, Andolina GG, Hamer DH, Leka LL, et al. Long-term care facilities: a cornucopia of viral pathogens. J Am Geriatr Soc. 2008; 56(7):1281–5.4. Birch CJ, Clothier HJ, Seccull A, Tran T, Catton MC, Lambert SB, et al. Human coronavirus OC43 causes influenza-like illness in residents and staff of aged-care facilities in Melbourne, Australia. Epidemiol Infect. 2005; 133(2):273–7.5. Walsh EE, Shin JH, Falsey AR. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis. 2013; 208(10):1634–42.6. Ko FW, Ip M, Chan PK, Chan MC, To KW, Ng SS, et al. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007; 132(3):900–8.7. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008; 134(6):1141–8.8. Krause JC, Panning M, Hengel H, Henneke P. The role of multiplex PCR in respiratory tract infections in children. Dtsch Arztebl Int. 2014; 111(38):639–45.9. Cherry JD, Krogstad P. SARS: the first pandemic of the 21st century. Pediatr Res. 2004; 56(1):1–5.10. Centers for Disease C, Prevention. Preliminary clinical description of severe acute respiratory syndrome. MMWR Morb Mortal Wkly Rep. 2003; 52(12):255–6.11. Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003; 349(25):2431–41.12. Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003; 348(20):1977–85.13. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012; 367(19):1814–20.14. Guery B, Poissy J, el Mansouf L, Sejourne C, Ettahar N, Lemaire X, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013; 381(9885):2265–72.15. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013; 13(9):752–61.16. Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013; 369(5):407–16.17. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015; 386(9997):995–1007.18. Deng SQ, Peng HJ. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J Clin Med. 2020; 9(2):19. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020; 63(3):457–60.20. Lam TT, Shum MH, Zhu HC, Tong YG, Ni XB, Liao YS, et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020.21. Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020; 11(1):1620.22. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020; 181(2):281–92. e6.23. Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016; 531(7592):118–21.24. Tresoldi I, Sangiuolo CF, Manzari V, Modesti A. SARS-COV-2 and infectivity. J Med Virol. 2020.25. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). In StatPearls. Treasure Island (FL);2020.26. WHO. Coronavirus disease (COVID-19) outbreak situation. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 2020.27. Central disease control headquaters. COVID-19 cases in Korea. http://ncov.mohw.go.kr/en/ 2020.28. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020; 5(4):335–7.29. Hindson J. COVID-19: faecal-oral transmission? Nat Rev Gastroenterol Hepatol. 2020.30. COVID-19 National Emergency Response Center EaCMT; Korea Centers for Disease Control and Prevention. Coronavirus Disease-19: Summary of 2,370 Contact Investigations of the First 30 Cases in the Republic of Korea. Osong Public Health and Research Perspectives. 2020; 11(2):81–4.31. Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020; 27(2):32. Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev. 1993; 15(2):265–302.33. Luman ET, Barker LE, Simpson DM, Rodewald LE, Szilagyi PG, Zhao Z. National, state and urban-area vaccination-coverage levels among children aged 19-35 months, United States, 1999. Am J Prev Med. 2001; 20(4 Suppl):88–153.34. Jiles RB, Fuchs C, Klevens RM. Vaccination coverage among children enrolled in Head Start programs or day care facilities or entering school. MMWR CDC Surveill Summ. 2000; 49(9):27–38.35. Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020.36. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020.37. Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARSCoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020; 382(12):1177–9.38. UK E. Loss of sense of smell as marker of COVID-19 infection. https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf 2020.39. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020.40. COVID-19 National Emergency Response Center EaCMT; Korea Centers for Disease Control and Prevention. Coronavirus Disease-19: The First 7,755 Cases in the Republic of Korea. Osong Public Health and Research Perspectives. 2020; 11(2):85–90.41. Choi E, Ha KS, Song DJ, Lee JH, Lee KC. Clinical and laboratory profiles of hospitalized children with acute respiratory virus infection. Korean J Pediatr. 2018; 61(6):180–6.42. Touzard-Romo F, Tape C, Lonks JR. Co-infection with SARS-CoV-2 and Human Metapneumovirus. R I Med J (2013). 2020; 103(2):75–6.43. Wahidi MM, Lamb C, Murgu S, Musani A, Shojaee S, Sachdeva A, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients with Suspected or Confirmed COVID-19 Infection. J Bronchology Interv Pulmonol. 2020.44. Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin Infect Dis. 2020.45. Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability Issues of RT-PCR Testing of SARS-CoV-2 for Hospitalized Patients Clinically Diagnosed with COVID-19. J Med Virol. 2020.46. Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J Med Virol. 2020.47. Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J Med Virol. 2020.48. Pedersen SF, Ho YC. SARS-CoV-2: A Storm is Raging. J Clin Invest. 2020.49. Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID-19): Analysis of Nine Patients Treated in Korea. Korean J Radiol. 2020; 21(4):494–500.50. Chua F, Armstrong-James D, Desai SR, Barnett J, Kouranos V, Kon OM, et al. The role of CT in case ascertainment and management of COVID-19 pneumonia in the UK: insights from high-incidence regions. Lancet Respir Me. 2020.51. Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhan N, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiolog. 2020:200463.52. Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiolog. 2020:200527.53. Liu KC, Xu P, Lv WF, Qiu XH, Yao JL, Gu JF, et al. CT manifestations of coronavirus disease-2019: A retrospective analysis of 73 cases by disease severity. Eur J Radiol. 2020; 126:108941.54. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiolog. 2020:200370.55. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiolog. 2020:200642.56. Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020; 15(3):e0230548.57. Liang T. Handbook of COVID-19 Prevention and Treatment. The First Affiliated Hospital, Zhejiang University School of Medicine. Complied According to Clinical Experience. 2020.58. Wu J, Li W, Shi X, Chen Z, Jiang B, Liu J, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. 2020.59. Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020.60. Zhao ZW, Zhang FC, Xu M, Huang K, Zhong WN, Cai WP, et al. [Clinical analysis of 190 cases of outbreak with atypical pneumonia in Guangzhou in spring, 2003]. Zhonghua Yi Xue Za Zhi. 2003; 83(9):713–8.61. Meng QH, Dong PL, Guo YB, Zhang K, Liang LC, Hou W, et al. [Use of glucocorticoid in treatment of severe acute respiratory syndrome cases]. Zhonghua Yu Fang Yi Xue Za Zhi. 2003; 37(4):233–5.62. Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020; 7(1):4.63. Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020:117583.64. Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020; 368:m1086.65. Rabito EI, Marcadenti A, da Silva Fink J, Figueira L, Silva FM. Nutritional Risk Screening 2002, Short Nutritional Assessment Questionnaire, Malnutrition Screening Tool, and Malnutrition Universal Screening Tool Are Good Predictors of Nutrition Risk in an Emergency Service. Nutr Clin Pract. 2017; 32(4):526–32.66. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020; 130(4):1545–8.67. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020.68. Mitja O, Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health. 2020.69. de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020; 117(12):6771–6.70. Sarma P, Kaur H, Kumar H, Mahendru D, Avti P, Bhattacharyya A, et al. Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis. J Med Virol. 2020.71. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020.72. WHO. “Solidarity” clinical trial for COVID-19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments. 2020.73. Leon Caly JDD, Mike G. Catton, David A. Jans, Kylie M. Wagstaff. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research 2020; In press.74. Prevention HPCfDCa. A Phase I Clinical Trial in 18-60 Adults (APICTH). Hubei Provincial Center for Disease Control and Prevention. https://clinicaltrials.gov/ct2/show/NCT04313127. 2020.75. Oxford Uo. A Study of a Candidate COVID-19 Vaccine (COV001). University of Oxford. https://clinicaltrials.gov/ct2/show/NCT04324606. 2020.76. Institute MCR. BCG Vaccination to Protect Healthcare Workers Against COVID-19 (BRACE). Murdoch Childrens Research Institute. https://clinicaltrials.gov/ct2/show/NCT04327206. 2020.77. NIH. NIH ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?recrs=e&cond=COVID-19 2020.78. Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020.79. Liu X, Zhang S. COVID-19: Face Masks and Human-to-human Transmission. Influenza Other Respir Viruses. 2020.80. Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020; 8(5):434–6.81. Schweon SJ, Edmonds SL, Kirk J, Rowland DY, Acosta C. Effectiveness of a comprehensive hand hygiene program for reduction of infection rates in a long-term care facility. Am J Infect Control. 2013; 41(1):39–44.82. Lewnard JA, Lo NC. Scientific and ethical basis for social-distancing interventions against COVID-19. Lancet Infect Dis. 2020.83. Koo JR, Cook AR, Park M, Sun Y, Sun H, Lim JT, et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect Dis. 2020.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Practical Recommendation of COVID-19 for Otorhinolaryngologists
- Contemporary Review of Olfactory Dysfunction in COVID-19
- The Potential Role of Dyslipidemia in COVID-19 Severity: an Umbrella Review of Systematic Reviews
- The Management of Thyroid Disease in COVID-19 Pandemic
- Assessment and Management of Dysphagia during the COVID-19 Pandemic