Int J Stem Cells.
2021 Feb;14(1):33-46. 10.15283/ijsc20023.
The Effect of L-Ascorbic Acid and Serum Reduction on Tenogenic Differentiation of Human Mesenchymal Stromal Cells
- Affiliations
-
- 1Department of Immunology, Transplantology and Internal Diseases, Medical University of Warsaw, Warsaw, Poland
- 2Department of Pharmacodynamics and Pathophysiology, Centre for Preclinical Research and Technology, Medical University of Warsaw, Warsaw, Poland
- 3Department of Physical Chemistry, Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Warsaw, Poland
- 4Department of Regenerative Medicine, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
- 5Department of Orthopedics and Traumatology, Medical University of Warsaw, Warsaw, Poland
- 6Department of Bioinformatics, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland
- KMID: 2513081
- DOI: http://doi.org/10.15283/ijsc20023
Abstract
- Background and Objectives
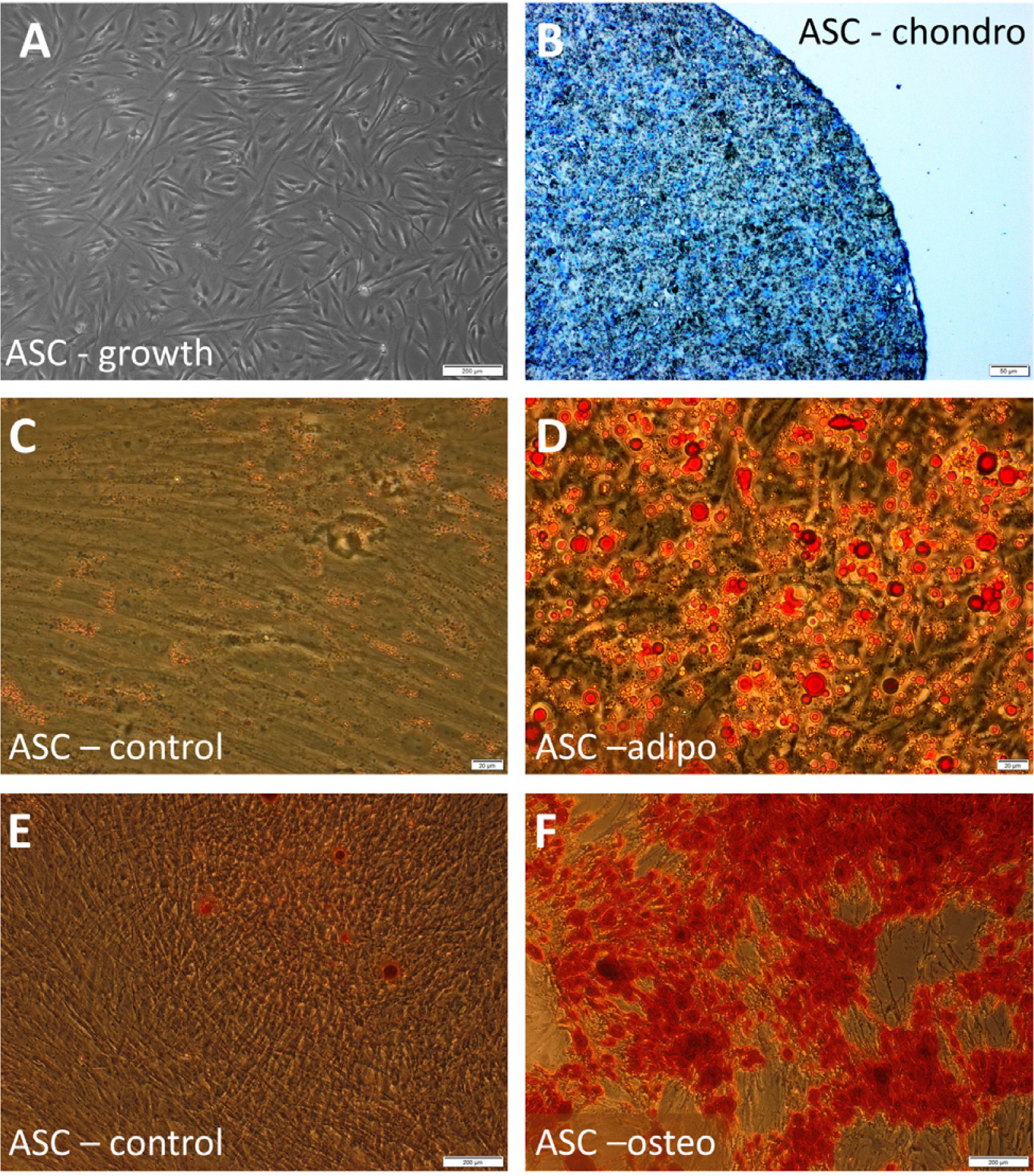
Despite significant improvement in the treatment of tendon injuries, the full tissue recovery is often not possible because of its limited ability to auto-repair. The transplantation of mesenchymal stromal cells (MSCs) is considered as a novel approach in the treatment of tendinopathies. The question about the optimal culture conditions remains open. In this study we aimed to investigate if serum reduction, L-ascorbic acid supplementation or a combination of both factors can induce tenogenic differentiation of human adipose-derived MSCs (ASCs).
Methods and Results
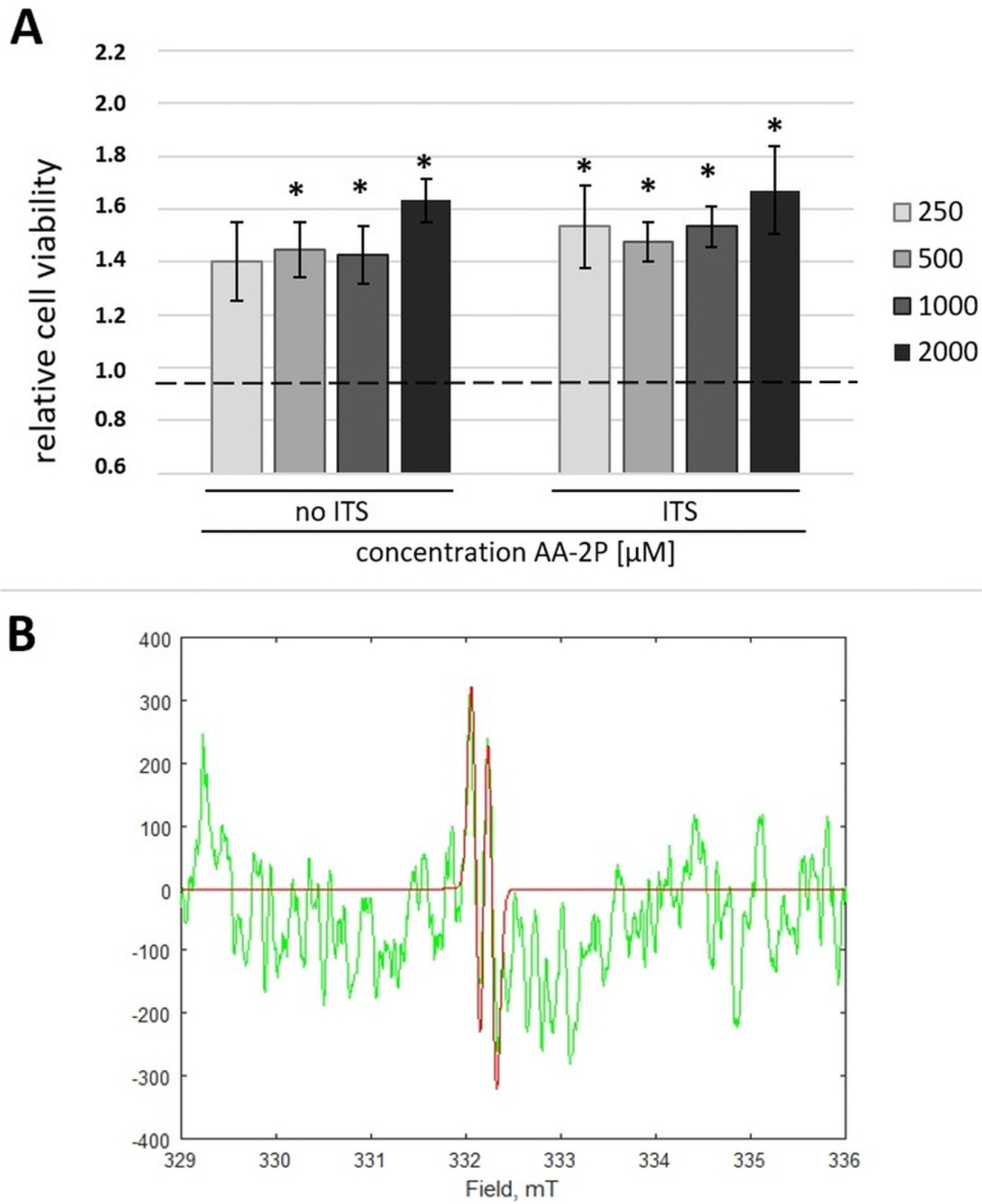
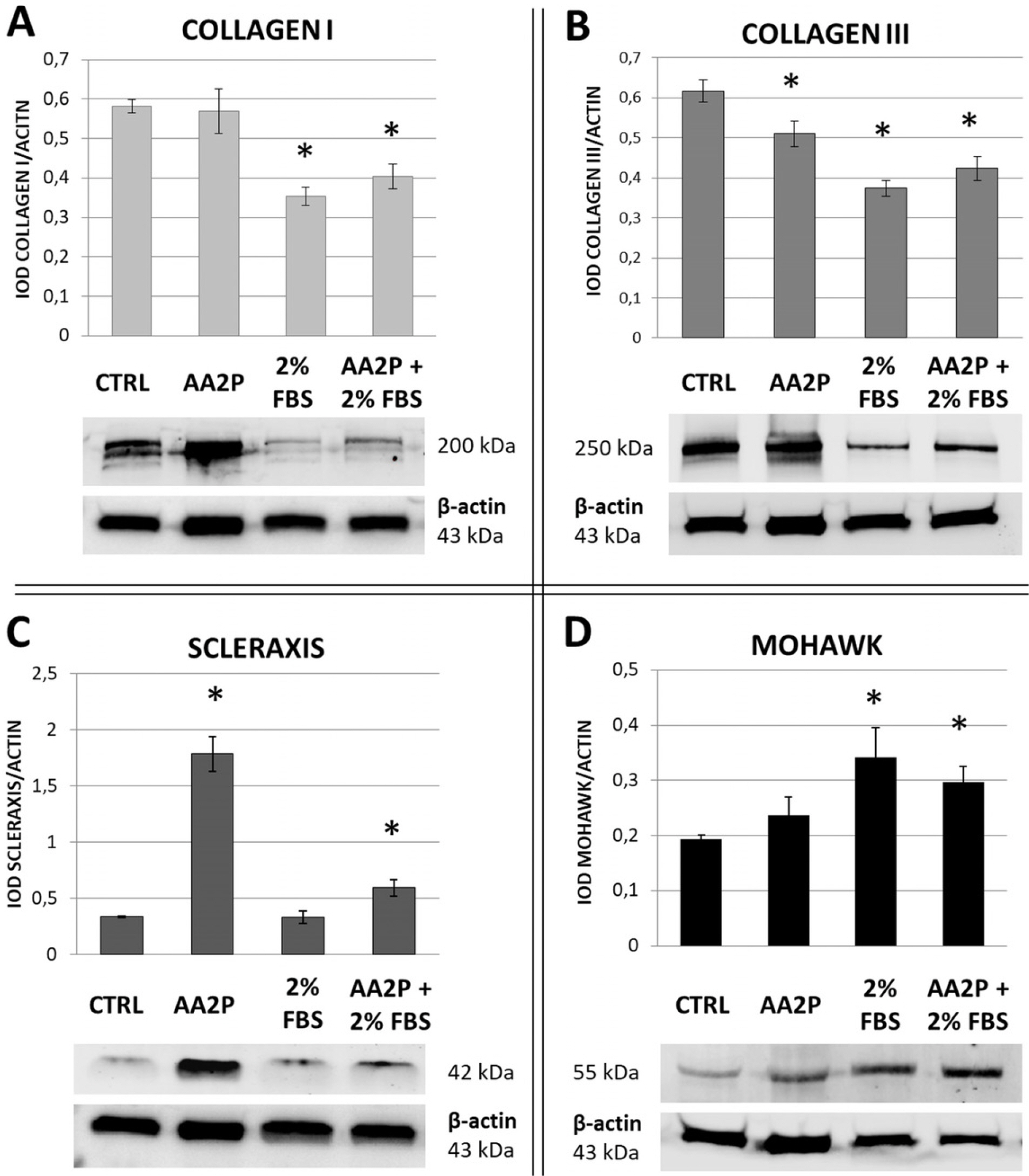
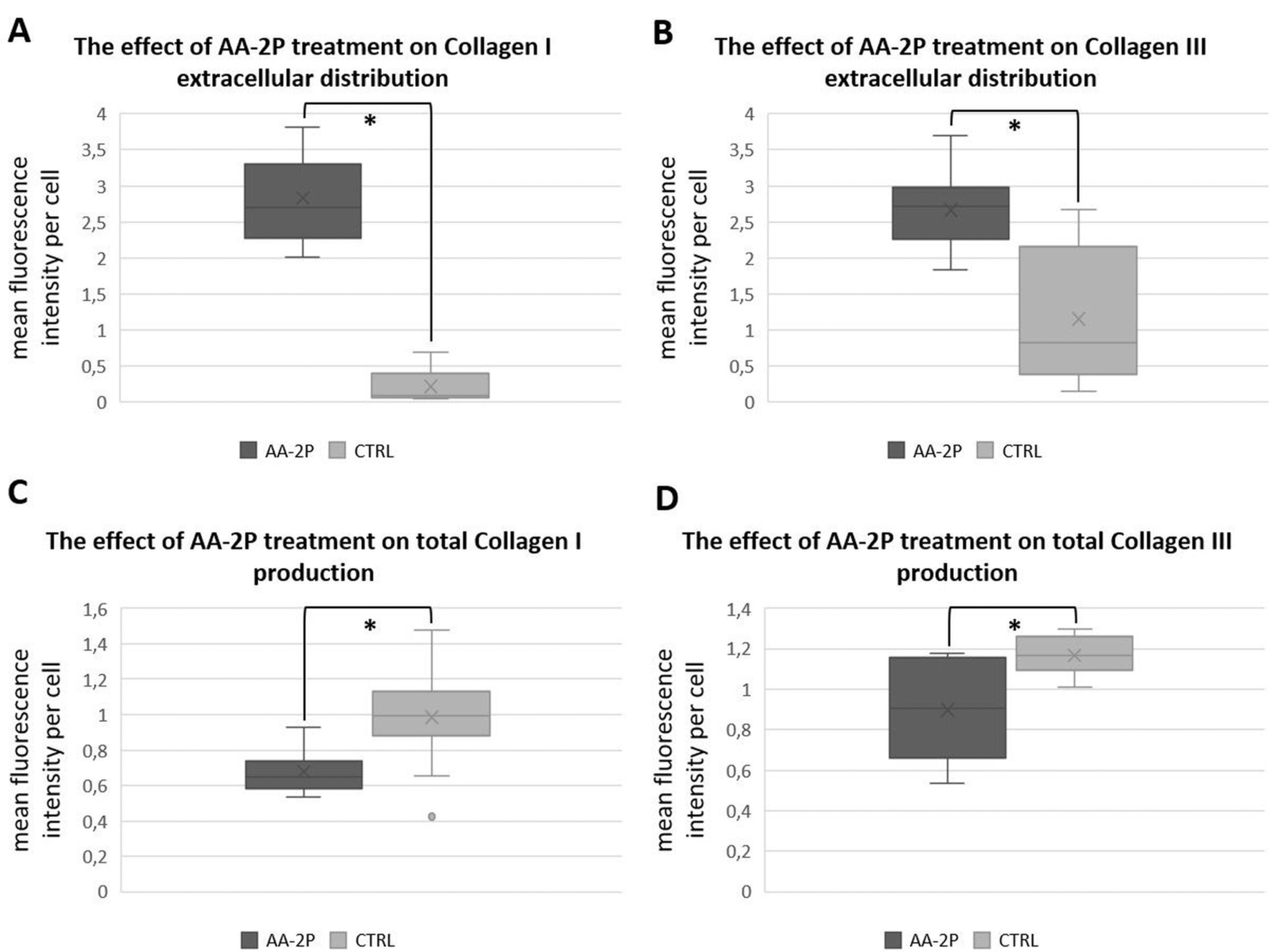
Human ASCs from 3 healthy donors were used in the study. The tested conditions were: 0.5 mM of ascorbic acid 2-phosphate (AA-2P), reduced serum content (2% FBS) or combination of these two factors. The combination of AA-2P and 2% FBS was the only experimental condition that caused a significant increase of the expression of all analyzed genes related to tenogenesis (SCLERAXIS, MOHAWK, COLLAGEN_1, COLLAGEN_3, DECORIN) in comparison to the untreated control (evaluated by RT-PCR, 5th day of experiment). Moreover, this treatment significantly increased the synthesis of SCLERAXIS, MOHAWK, COLLAGEN_1, COLLAGEN_3 proteins at the same time point (evaluated by Western blot method). Double immunocytochemical staining revealed that AA-2P significantly increased the extracellular deposition of both types of collagens. Semi-quantitative Electron Spin Resonance analysis of ascorbyl free radical revealed that AA-2P do not induce harmful transition metals-driven redox reactions in cell culture media.
Conclusions
Obtained results justify the use of reduced content of serum with the addition of 0.5 mM of AA-2P in tenogenic inducing media.
Figure
Reference
-
References
1. Dakin SG, Dudhia J, Smith RK. 2014; Resolving an inflamma-tory concept: the importance of inflammation and resolution in tendinopathy. Vet Immunol Immunopathol. 158:121–127. DOI: 10.1016/j.vetimm.2014.01.007. PMID: 24556326. PMCID: PMC3991845.
Article2. Aicale R, Tarantino D, Maffulli N. 2018; Overuse injuries in sport: a comprehensive overview. J Orthop Surg Res. 13:309. DOI: 10.1186/s13018-018-1017-5. PMID: 30518382. PMCID: PMC6282309.
Article3. Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. 2015; Mechanisms of tendon injury and repair. J Orthop Res. 33:832–839. DOI: 10.1002/jor.22806. PMID: 25641114. PMCID: PMC4418182.
Article4. Yan Z, Yin H, Nerlich M, Pfeifer CG, Docheva D. 2018; Boosting tendon repair: interplay of cells, growth factors and scaffold-free and gel-based carriers. J Exp Orthop. 5:1. DOI: 10.1186/s40634-017-0117-1. PMID: 29330711. PMCID: PMC5768579.
Article5. Lui PP, Ng SW. 2013; Cell therapy for the treatment of tendinopathy--a systematic review on the pre-clinical and clinical evidence. Semin Arthritis Rheum. 42:651–666. DOI: 10.1016/j.semarthrit.2012.10.004. PMID: 23369658.
Article6. Schmitt A, van Griensven M, Imhoff AB, Buchmann S. 2012; Application of stem cells in orthopedics. Stem Cells Int. 2012:394962. DOI: 10.1155/2012/394962. PMID: 22550505. PMCID: PMC3328166.
Article7. Lee SY, Kwon B, Lee K, Son YH, Chung SG. 2017; Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 45:1429–1439. DOI: 10.1177/0363546517689874. PMID: 28291954.
Article8. Liu Y, Suen CW, Zhang JF, Li G. 2017; Current concepts on tenogenic differentiation and clinical applications. J Orthop Translat. 9:28–42. DOI: 10.1016/j.jot.2017.02.005. PMID: 29662797. PMCID: PMC5822963.
Article9. Bottagisio M, Lopa S, Granata V, Talò G, Bazzocchi C, Moretti M, et al. 2017; Different combinations of growth factors for the tenogenic differentiation of bone marrow mesenchymal stem cells in monolayer culture and in fibrin-based three-dimensional constructs. Differentiation. 95:44–53. DOI: 10.1016/j.diff.2017.03.001. PMID: 28319735.
Article10. Yu Y, Zhou Y, Cheng T, Lu X, Yu K, Zhou Y, Hong J, Chen Y. 2016; Hypoxia enhances tenocyte differentiation of adipose-derived mesenchymal stem cells by inducing hypoxia- inducible factor-1α in a co-culture system. Cell Prolif. 49:173–184. DOI: 10.1111/cpr.12250. PMID: 27021233. PMCID: PMC6496829.
Article11. Veronesi F, Torricelli P, Della Bella E, Pagani S, Fini M. 2015; In vitro mutual interaction between tenocytes and adipose- derived mesenchymal stromal cells. Cytotherapy. 17:215–223. DOI: 10.1016/j.jcyt.2014.10.006. PMID: 25484309.
Article12. Popov C, Burggraf M, Kreja L, Ignatius A, Schieker M, Docheva D. 2015; Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol Biol. 16:6. DOI: 10.1186/s12867-015-0036-6. PMID: 25880261. PMCID: PMC4373449.
Article13. Rinoldi C, Costantini M, Kijeńska-Gawrońska E, Testa S, Fornetti E, Heljak M, Ćwiklińska M, Buda R, Baldi J, Cannata S, Guzowski J, Gargioli C, Khademhosseini A, Swieszkowski W. 2019; Tendon tissue engineering: effects of mechanical and biochemical stimulation on stem cell alignment on cell-laden hydrogel yarns. Adv Healthc Mater. 8:e1801218. DOI: 10.1002/adhm.201801218. PMID: 30725521.
Article14. Dyment NA, Galloway JL. 2015; Regenerative biology of tendon: mechanisms for renewal and repair. Curr Mol Biol Rep. 1:124–131. DOI: 10.1007/s40610-015-0021-3. PMID: 26389023. PMCID: PMC4570727.
Article15. Lui PP, Wong OT, Lee YW. 2016; Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytothe-rapy. 18:99–112. DOI: 10.1016/j.jcyt.2015.10.005. PMID: 26719200.
Article16. Stanco D, Caprara C, Ciardelli G, Mariotta L, Gola M, Minonzio G, Soldati G. 2019; Tenogenic differentiation protocol in xenogenic-free media enhances tendon-related marker expression in ASCs. PLoS One. 14:e0212192. DOI: 10.1371/journal.pone.0212192. PMID: 30753235. PMCID: PMC6372228.
Article17. Perucca Orfei C, Viganò M, Pearson JR, Colombini A, De Luca P, Ragni E, Santos-Ruiz L, de Girolamo L. 2019; In vitro induction of tendon-specific markers in tendon cells, adipose- and bone marrow-derived stem cells is dependent on TGFβ3, BMP-12 and ascorbic acid stimulation. Int J Mol Sci. 20:149. DOI: 10.3390/ijms20010149. PMID: 30609804. PMCID: PMC6337430.
Article18. Moores J. 2013; Vitamin C: a wound healing perspective. Br J Community Nurs. Suppl:S6S8–S11. DOI: 10.12968/bjcn.2013.18.Sup12.S6. PMID: 24796079.
Article19. Michels AJ, Frei B. 2013; Myths, artifacts, and fatal flaws: identifying limitations and opportunities in vitamin C research. Nutrients. 5:5161–5192. DOI: 10.3390/nu5125161. PMID: 24352093. PMCID: PMC3875932.
Article20. Zarychta-Wiśniewska W, Burdzinska A, Kulesza A, Gala K, Kaleta B, Zielniok K, Siennicka K, Sabat M, Paczek L. 2017; Bmp-12 activates tenogenic pathway in human adipose stem cells and affects their immunomodulatory and secretory properties. BMC Cell Biol. 18:13. DOI: 10.1186/s12860-017-0129-9. PMID: 28214472. PMCID: PMC5316159.
Article21. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012; Fiji: an open-source platform for biological-image analysis. Nat Methods. 9:676–682. DOI: 10.1038/nmeth.2019. PMID: 22743772. PMCID: PMC3855844.
Article22. Vergely C, Maupoil V, Clermont G, Bril A, Rochette L. 2003; Identification and quantification of free radicals during myocardial ischemia and reperfusion using electron paramagnetic resonance spectroscopy. Arch Biochem Biophys. 420:209–216. DOI: 10.1016/j.abb.2003.07.007. PMID: 14654059.
Article23. Dale TP, Mazher S, Webb WR, Zhou J, Maffulli N, Chen GQ, et al. 2018; Tenogenic differentiation of human embryonic stem cells. Tissue Eng Part A. 24:361–368. DOI: 10.1089/ten.tea.2017.0017. PMID: 28548630.
Article24. Viganò M, Perucca Orfei C, de Girolamo L, Pearson JR, Ragni E, De Luca P, Colombini A. 2018; Housekeeping gene stability in human mesenchymal stem and tendon cells exposed to tenogenic factors. Tissue Eng Part C Methods. 24:360–367. DOI: 10.1089/ten.tec.2017.0518. PMID: 29676207.
Article25. Stanco D, Viganò M, Perucca Orfei C, Di Giancamillo A, Peretti GM, Lanfranchi L, de Girolamo L. 2015; Multidifferen-tiation potential of human mesenchymal stem cells from adipose tissue and hamstring tendons for musculoskeletal cell-based therapy. Regen Med. 10:729–743. DOI: 10.2217/rme.14.92. PMID: 25565145.
Article26. Tikekar RV, Anantheswaran RC, Elias RJ, LaBorde LF. 2011; Ultraviolet-induced oxidation of ascorbic acid in a model juice system: identification of degradation products. J Agric Food Chem. 59:8244–8248. DOI: 10.1021/jf201000x. PMID: 21699245.
Article27. Takamizawa S, Maehata Y, Imai K, Senoo H, Sato S, Hata R. 2004; Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol Int. 28:255–265. DOI: 10.1016/j.cellbi.2004.01.010. PMID: 15109981.
Article28. Zarychta-Wiśniewska W, Burdzińska A, Zielniok K, Koblowska M, Gala K, Pędzisz P, Iwanicka-Nowicka R, Fogtman A, Aksamit A, Kulesza A, Zołocińska A, Pączek L. 2019; The influence of cell source and donor age on the tenogenic potential and chemokine secretion of human mesenchymal stromal cells. Stem Cells Int. 2019:1613701. DOI: 10.1155/2019/1613701. PMID: 31205472. PMCID: PMC6530320.
Article29. Kang KK, Lee EJ, Kim YD, Chung MJ, Kim JY, Kim SY, Hwang SK, Jeong KS. 2017; Vitamin C improves therapeutic effects of adipose-derived stem cell transplantation in mouse tendonitis model. In Vivo. 31:343–348. DOI: 10.21873/invivo.11065. PMID: 28438861. PMCID: PMC5461443.
Article30. Sassoon AA, Ozasa Y, Chikenji T, Sun YL, Larson DR, Maas ML, Zhao C, Jen J, Amadio PC. 2012; Skeletal muscle and bone marrow derived stromal cells: a comparison of tenocyte differentiation capabilities. J Orthop Res. 30:1710–1718. DOI: 10.1002/jor.22135. PMID: 22511232. PMCID: PMC3402710.
Article31. Theiss F, Mirsaidi A, Mhanna R, Kümmerle J, Glanz S, Bahrenberg G, Tiaden AN, Richards PJ. 2015; Use of biomimetic microtissue spheroids and specific growth factor supplementation to improve tenocyte differentiation and adaptation to a collagen-based scaffold in vitro. Biomaterials. 69:99–109. DOI: 10.1016/j.biomaterials.2015.08.013. PMID: 26283157.
Article32. Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD. 2014; Nutrient-sensing nuclear receptors coordinate autophagy. Nature. 516:112–115. DOI: 10.1038/nature13961. PMID: 25383539. PMCID: PMC4267857.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Coculture Effects on the Osteogenic Differentiation of Human Mesenchymal Stromal Cells
- The Concentration of Ascorbic acid in Vitreous of Macular Hole Patients
- Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?
- An Implant for Bone Formation using Autologous Mesenchymal Stem Cells
- Effects of Ascorbic acid on the Intraocular Pressure and Serum Osmolarity in Rabbits