Therapeutic Effects of Fibroblast Growth Factor-21 on Diabetic Nephropathy and the Possible Mechanism in Type 1 Diabetes Mellitus Mice
- Affiliations
-
- 1Ruian Center of the Chinese-American Institute for Diabetic Complications, the Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, China.
- 2Cancer Center, the First Hospital of Jilin University, Changchun, China.
- 3Biological Engineering Department, School of Life Science, Anhui Medical University, Hefei, China.
- 4The Chinese-American Research Institute for Diabetic Complications, Wenzhou Medical University, Wenzhou, China.
- KMID: 2513016
- DOI: http://doi.org/10.4093/dmj.2019.0089
Abstract
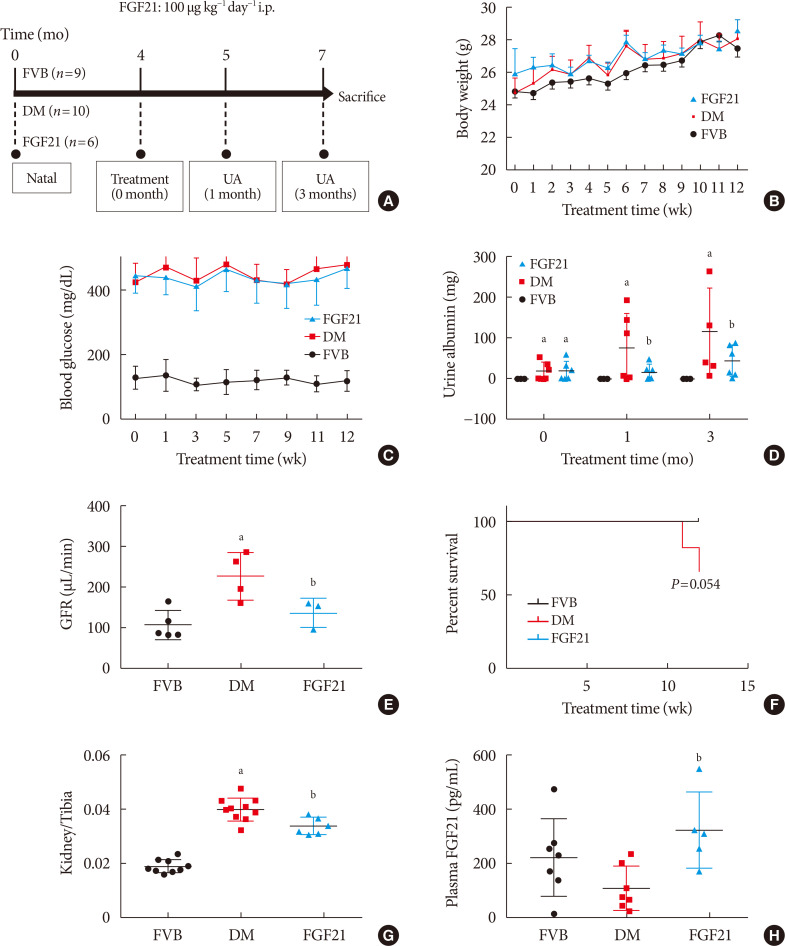
Background Fibroblast growth factor 21 (FGF21) has been only reported to prevent type 1 diabetic nephropathy (DN) in the streptozotocin-induced type 1 diabetes mellitus (T1DM) mouse model. However, the FVB (Cg)-Tg (Cryaa-Tag, Ins2-CALM1) 26OVE/PneJ (OVE26) transgenic mouse is a widely recommended mouse model to recapture the most important features of T1DM nephropathy that often occurs in diabetic patients. In addition, most previous studies focused on exploring the preventive effect of FGF21 on the development of DN. However, in clinic, development of therapeutic strategy has much more realistic value compared with preventive strategy since the onset time of DN is difficult to be accurately predicted. Therefore, in the present study OVE26 mice were used to investigate the potential therapeutic effects of FGF21 on DN.
Methods Four-month-old female OVE26 mice were intraperitoneally treated with recombinant FGF21 at a dose of 100 µg/kg/day for 3 months. The diabetic and non-diabetic control mice were treated with phosphate-buffered saline at the same volume. Renal functions, pathological changes, inflammation, apoptosis, oxidative stress and fibrosis were examined in mice of all groups.
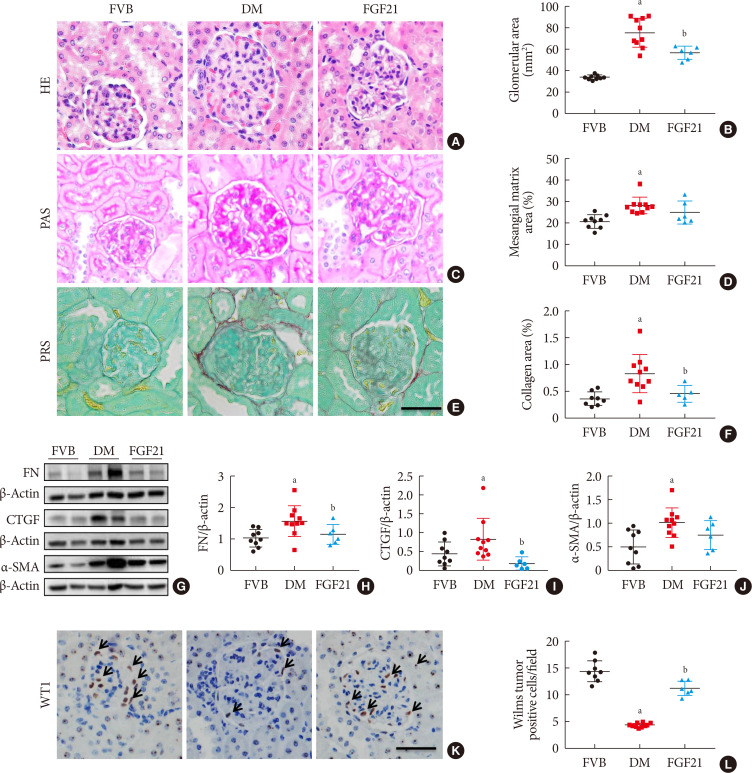
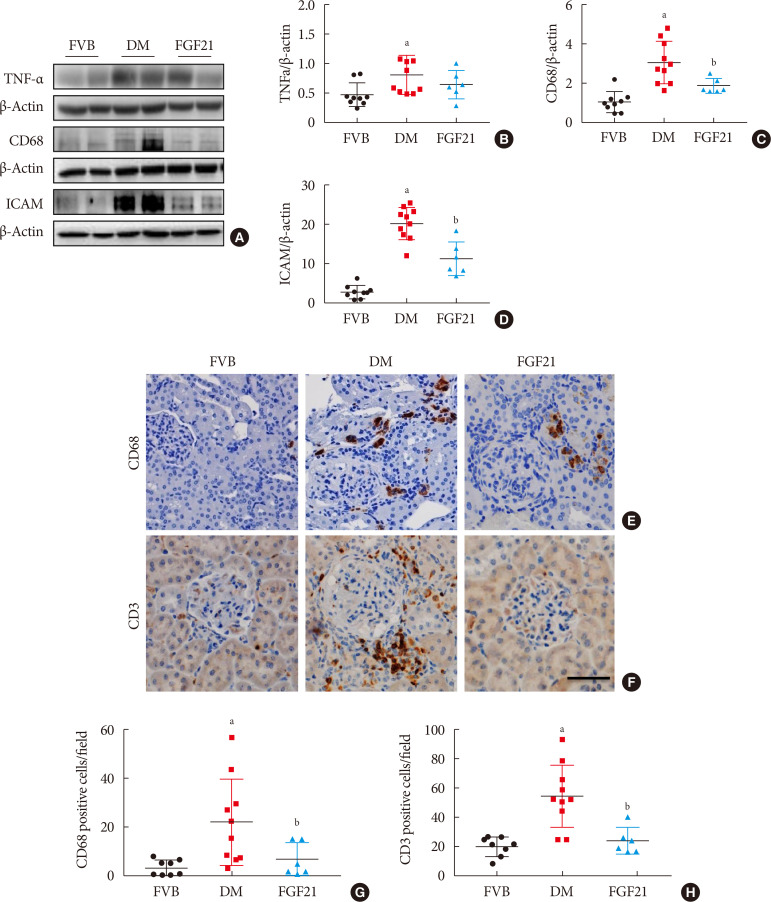
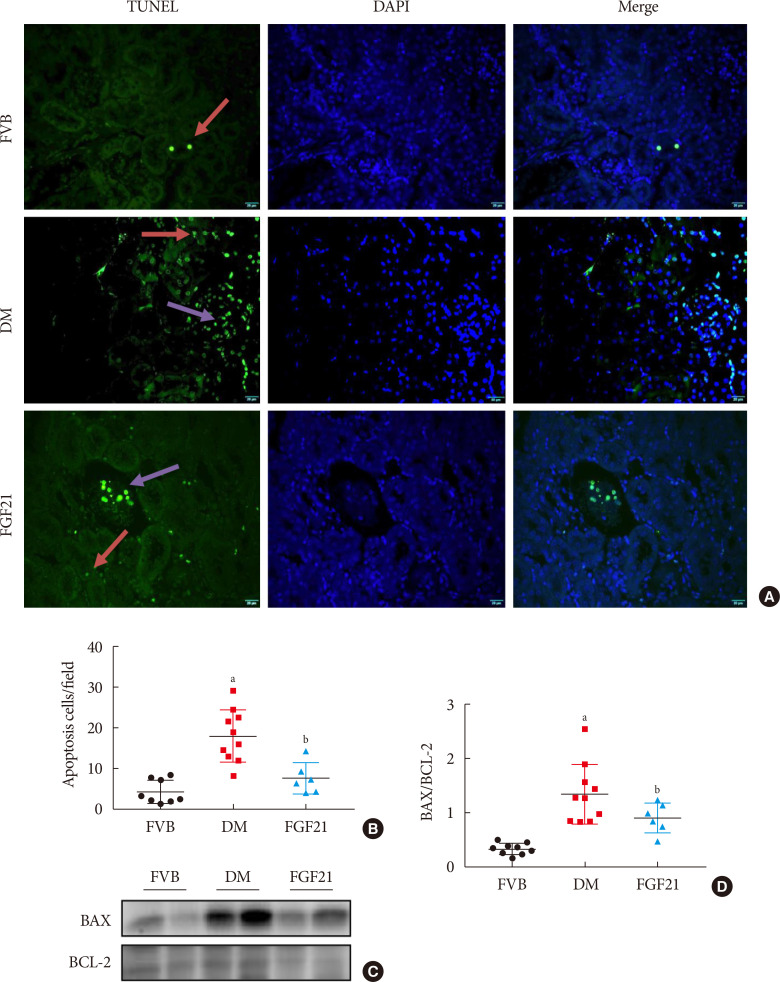
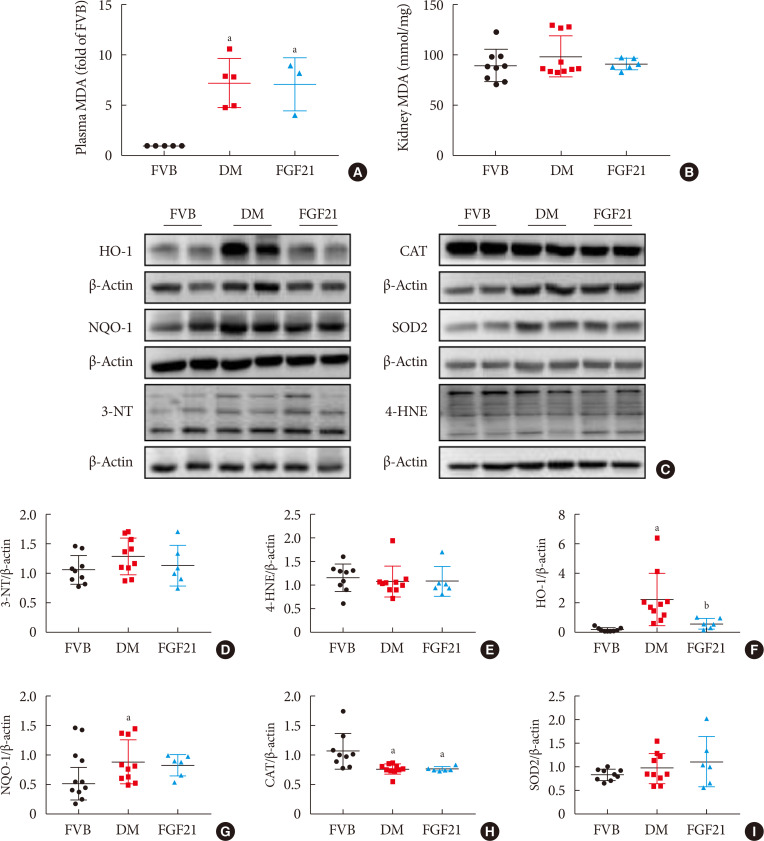
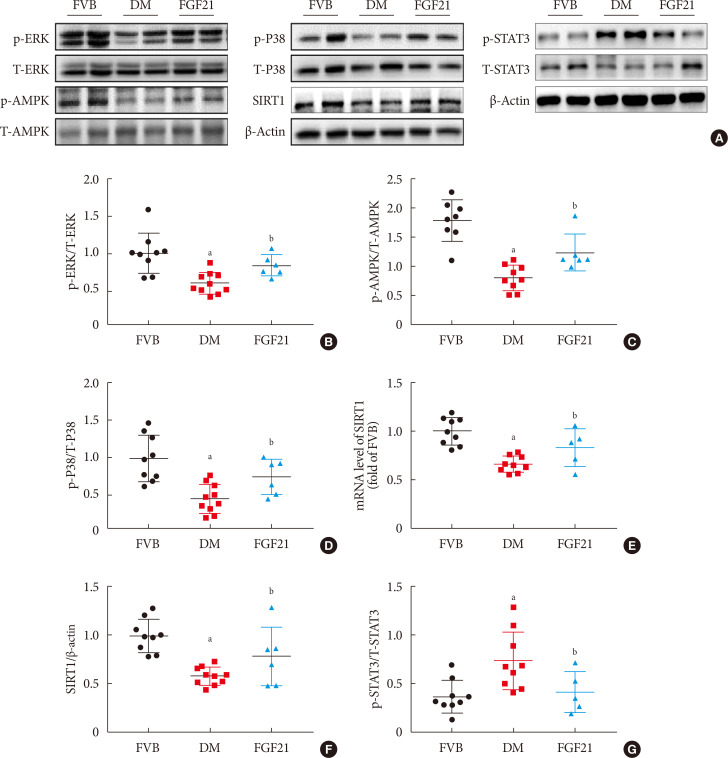
Results The results showed that severe renal dysfunction, morphological changes, inflammation, apoptosis, and fibrosis were observed in OVE26 mice. However, all the renal abnormalities above in OVE26 mice were significantly attenuated by 3-month FGF21 treatment associated with improvement of renal adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) activity and sirtuin 1 (SIRT1) expression.
Conclusion Therefore, this study demonstrated that FGF21 might exert therapeutic effects on DN through AMPK-SIRT1 pathway.
Keyword
Figure
Reference
-
1. Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O'Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang D, Wang M, Woodside KJ, Xin X, Yin M, You AS, Zhou H, Shahinian V. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018; 71(3 Suppl 1):A7. PMID: 29477157.
Article2. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Appleyard M, Jensen JS. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004; 110:32–35. PMID: 15210602.
Article3. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005; 115:1627–1635. PMID: 15902306.
Article4. El-Saeed AM, El-Mohasseb GF. Circulating fibroblast growth factors 21 and 23 as biomarkers of progression in diabetic nephropathy in type 2 diabetes with normoalbuminuria. Egypt J Immunol. 2017; 24:93–99. PMID: 29528583.5. Esteghamati A, Khandan A, Momeni A, Behdadnia A, Ghajar A, Nikdad MS, Noshad S, Nakhjavani M, Afarideh M. Circulating levels of fibroblast growth factor 21 in early-stage diabetic kidney disease. Ir J Med Sci. 2017; 186:785–794. PMID: 28181108.
Article6. Wei W, An XR, Jin SJ, Li XX, Xu M. Inhibition of insulin resistance by PGE1 via autophagy-dependent FGF21 pathway in diabetic nephropathy. Sci Rep. 2018; 8:9. PMID: 29311680.
Article7. Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, Lee MH, Song HK, Nam DH, Han JY, Han SY, Han KH, Kang YS, Cha DR. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology. 2013; 154:3366–3376. PMID: 23825123.
Article8. Shao M, Yu L, Zhang F, Lu X, Li X, Cheng P, Lin X, He L, Jin S, Tan Y, Yang H, Zhang C, Cai L. Additive protection by LDR and FGF21 treatment against diabetic nephropathy in type 2 diabetes model. Am J Physiol Endocrinol Metab. 2015; 309:E45–E54. PMID: 25968574.
Article9. Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010; 77:57–64. PMID: 19847154.
Article10. Kim WH, Lee JW, Suh YH, Lee HJ, Lee SH, Oh YK, Gao B, Jung MH. AICAR potentiates ROS production induced by chronic high glucose: roles of AMPK in pancreatic beta-cell apoptosis. Cell Signal. 2007; 19:791–805. PMID: 17127032.11. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8:1288–1295. PMID: 12368907.
Article12. Papadimitriou A, Peixoto EB, Silva KC, Lopes de Faria JM, Lopes de Faria JB. Increase in AMPK brought about by cocoa is renoprotective in experimental diabetes mellitus by reducing NOX4/TGFβ-1 signaling. J Nutr Biochem. 2014; 25:773–784. PMID: 24768660.
Article13. Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing claudin-1 overexpression in podocytes. Nat Med. 2013; 19:1496–1504. PMID: 24141423.
Article14. Preyat N, Leo O. Sirtuin deacylases: a molecular link between metabolism and immunity. J Leukoc Biol. 2013; 93:669–680. PMID: 23325925.
Article15. Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010; 30:10220–10232. PMID: 20668205.
Article16. Papadimitriou A, Silva KC, Peixoto EB, Borges CM, Lopes de Faria JM, Lopes de Faria JB. Theobromine increases NAD+/Sirt-1 activity and protects the kidney under diabetic conditions. Am J Physiol Renal Physiol. 2015; 308:F209–F225. PMID: 25411384.17. Zhang C, Huang Z, Gu J, Yan X, Lu X, Zhou S, Wang S, Shao M, Zhang F, Cheng P, Feng W, Tan Y, Li X. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia. 2015; 58:1937–1948. PMID: 26040473.
Article18. Jiang X, Chen J, Zhang C, Zhang Z, Tan Y, Feng W, Skibba M, Xin Y, Cai L. The protective effect of FGF21 on diabetes-induced male germ cell apoptosis is associated with up-regulated testicular AKT and AMPK/Sirt1/PGC-1α signaling. Endocrinology. 2015; 156:1156–1170. PMID: 25560828.
Article19. Zhang J, Cheng Y, Gu J, Wang S, Zhou S, Wang Y, Tan Y, Feng W, Fu Y, Mellen N, Cheng R, Ma J, Zhang C, Li Z, Cai L. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of type 1 diabetic mice. Clin Sci (Lond). 2016; 130:625–641. PMID: 26795437.20. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018; 25:121–132. PMID: 29580576.
Article21. Zhang C, Shao M, Yang H, Chen L, Yu L, Cong W, Tian H, Zhang F, Cheng P, Jin L, Tan Y, Li X, Cai L, Lu X. Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS One. 2013; 8:e82275. PMID: 24349242.
Article22. Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004; 53:3248–3257. PMID: 15561957.
Article23. Xu Z, Tong Q, Zhang Z, Wang S, Zheng Y, Liu Q, Qian LB, Chen SY, Sun J, Cai L. Inhibition of HDAC3 prevents diabetic cardiomyopathy in OVE26 mice via epigenetic regulation of DUSP5-ERK1/2 pathway. Clin Sci (Lond). 2017; 131:1841–1857. PMID: 28533215.
Article24. Xu J, Huang Y, Li F, Zheng S, Epstein PN. FVB mouse genotype confers susceptibility to OVE26 diabetic albuminuria. Am J Physiol Renal Physiol. 2010; 299:F487–F494. PMID: 20610531.
Article25. Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005; 54:1829–1837. PMID: 15919806.
Article26. Cui W, Li B, Bai Y, Miao X, Chen Q, Sun W, Tan Y, Luo P, Zhang C, Zheng S, Epstein PN, Miao L, Cai L. Potential role for Nrf2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am J Physiol Endocrinol Metab. 2013; 304:E87–E99. PMID: 23132297.
Article27. Nerstedt A, Johansson A, Andersson CX, Cansby E, Smith U, Mahlapuu M. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3). Diabetologia. 2010; 53:2406–2416. PMID: 20652679.
Article28. Li H, Lee J, He C, Zou MH, Xie Z. Suppression of the mTORC1/STAT3/Notch1 pathway by activated AMPK prevents hepatic insulin resistance induced by excess amino acids. Am J Physiol Endocrinol Metab. 2014; 306:E197–E209. PMID: 24302004.
Article29. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003; 42:1206–1252. PMID: 14656957.
Article30. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008; 149:6018–6027. PMID: 18687777.
Article31. Mao S, Ren X, Zhang J. The emerging role of fibroblast growth factor 21 in diabetic nephropathy. J Recept Signal Transduct Res. 2016; 36:586–592. PMID: 26915669.
Article32. Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early-onset diabetes in transgenic mice. Cell. 1989; 58:1067–1073. PMID: 2673540.
Article33. Yoon SJ, Kim SJ, Lee SM. Overexpression of HO-1 contributes to sepsis-induced immunosuppression by modulating the Th1/Th2 balance and regulatory T-cell function. J Infect Dis. 2017; 215:1608–1618. PMID: 28368519.
Article34. Scharn CR, Collins AC, Nair VR, Stamm CE, Marciano DK, Graviss EA, Shiloh MU. Heme oxygenase-1 regulates inflammation and mycobacterial survival in human macrophages during mycobacterium tuberculosis infection. J Immunol. 2016; 196:4641–4649. PMID: 27183573.35. Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999; 13:1800–1809. PMID: 10506583.
Article36. Luo X, Deng L, Lamsal LP, Xu W, Xiang C, Cheng L. AMP-activated protein kinase alleviates extracellular matrix accumulation in high glucose-induced renal fibroblasts through mTOR signaling pathway. Cell Physiol Biochem. 2015; 35:191–200. PMID: 25591762.
Article37. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A. 2010; 107:12553–12558. PMID: 20616029.38. Chuang PY, Dai Y, Liu R, He H, Kretzler M, Jim B, Cohen CD, He JC. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One. 2011; 6:e23566. PMID: 21858169.
Article39. Chuang PY, Cai W, Li X, Fang L, Xu J, Yacoub R, He JC, Lee K. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am J Physiol Renal Physiol. 2017; 313:F621–F628. PMID: 28615249.
Article40. Yan J, Wang J, Huang H, Huang Y, Mi T, Zhang C, Zhang L. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H(2)O(2)-induced premature senescence through SIRT1. Am J Transl Res. 2017; 9:4492–4501. PMID: 29118911.41. Makela J, Tselykh TV, Maiorana F, Eriksson O, Do HT, Mudo G, Korhonen LT, Belluardo N, Lindholm D. Fibroblast growth factor-21 enhances mitochondrial functions and increases the activity of PGC-1α in human dopaminergic neurons via Sirtuin-1. Springerplus. 2014; 3:2. PMID: 25932355.
Article42. Wu H, Liu Y, Chen X, Zhu D, Ma J, Yan Y, Si M, Li X, Sun C, Yang B, He Q, Chen K. Neohesperidin exerts lipid-regulating effects in vitro and in vivo via fibroblast growth factor 21 and AMP-activated protein kinase/sirtuin type 1/peroxisome proliferator-activated receptor gamma coactivator 1α signaling axis. Pharmacology. 2017; 100:115–126. PMID: 28554169.
Article43. Liu X, Wang Y, Hou L, Xiong Y, Zhao S. Fibroblast growth factor 21 (FGF21) promotes formation of aerobic myofibers via the FGF21-SIRT1-AMPK-PGC1α pathway. J Cell Physiol. 2017; 232:1893–1906. PMID: 27966786.
Article44. Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, Tse HF, Chau MT, Cheung BM, Lam KS. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013; 33:2454–2459. PMID: 23887638.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fibroblast Growth Factor 21 Attenuates Diabetes-Induced Renal Fibrosis by Negatively Regulating TGF-β-p53-Smad2/3-Mediated Epithelial-to-Mesenchymal Transition via Activation of AKT
- Diabetic Nephropathy: New insights into the pathogenesis
- Diet Therapy in Patients of Diabetic Nephropathy
- Recent Updates on Diabetic Nephropathy
- Diabetic Nephropathy in Childhood and Adolescence (I) : Clinical Features