Ann Lab Med.
2021 Jul;41(4):424-428. 10.3343/alm.2021.41.4.424.
Laboratory Aspects of Donor Screening for Fecal Microbiota Transplantation at a Korean Fecal Microbiota Bank
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea
- 2Microbiotix Corporation, Seoul, Korea
- 3Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2512724
- DOI: http://doi.org/10.3343/alm.2021.41.4.424
Abstract
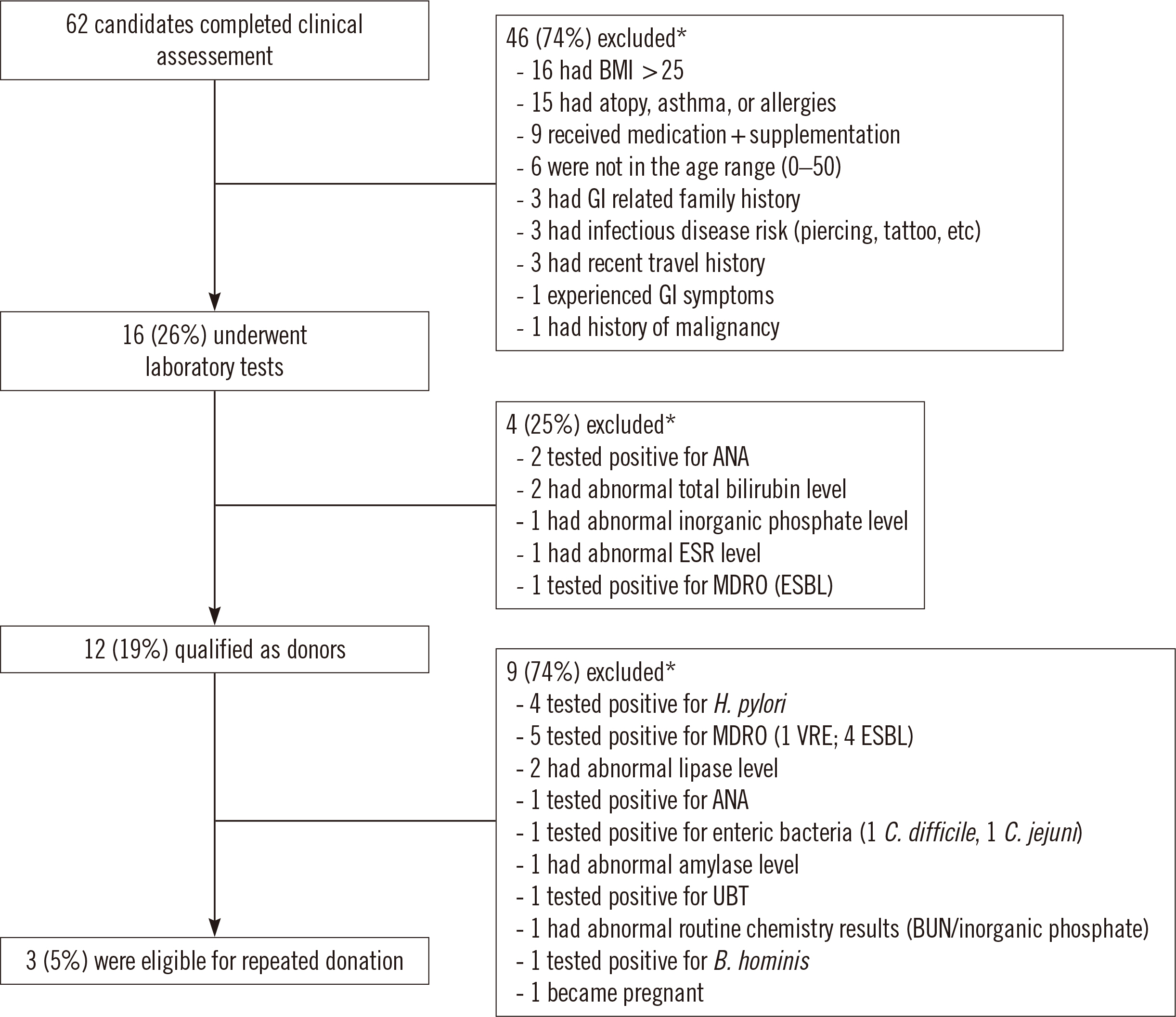
- Fecal microbiota transplantation (FMT) is a widely accepted alternative therapy for Clostridioides difficile infection and other gastrointestinal disorders. Thorough donor screening is required as a safety control measure to minimize transmission of infectious agents in FMT. We report the donor screening process and outcomes at a fecal microbiota bank in Korea. From August 2017 to June 2020, the qualification of 62 individuals as FMT donors was evaluated using clinical assessment and laboratory tests. Forty-six (74%) candidates were excluded after clinical assessment; high body mass index ( > 25) was the most common reason for exclusion, followed by atopy, asthma, and allergy history. Four of the remaining 16 (25%) candidates failed to meet laboratory test criteria, resulting in a 19% qualification rate. FMT donor re-qualification was conducted monthly as an additional safety control measure, and only three (5%) candidates were eligible for repeated donation. As high prevalence of multidrug-resistant organisms (55%) and Helicobacter pylori (44%) were detected in qualified donors during the screening, a urea breath test was added to the existing protocol. The present results emphasize the importance of implementing a donor re-qualification system to minimize risk factors not identified during initial donor screening.
Figure
Reference
-
1. Dinh A, Fessi H, Duran C, Batista R, Michelon H, Bouchand F, et al. 2018; Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect. 99:481–6. DOI: 10.1016/j.jhin.2018.02.018. PMID: 29477634.2. Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. 2017; Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 318:1985–93. DOI: 10.1001/jama.2017.17077. PMID: 29183074. PMCID: PMC5820695.3. Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, et al. 2020; Fecal microbiota transplantation to maintain remission in Crohn's disease: a pilot randomized controlled study. Microbiome. 8:12. DOI: 10.1186/s40168-020-0792-5. PMID: 32014035. PMCID: PMC6998149.
Article4. Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. 2017; European consensus conference on faecal microbiota transplant in clinical practice. Gut. 66:569–80. DOI: 10.1136/gutjnl-2016-313017. PMID: 28087657. PMCID: PMC5529972.5. Costello SP, Tucker EC, La Brooy J, Schoeman MN, Andrews JM. 2016; Establishing a fecal microbiota transplant service for the treatment of Clostridium difficile infection. Clin Infect Dis. 62:908–14. DOI: 10.1093/cid/civ994. PMID: 26628567.6. Craven LJ, Nair Parvathy S, Tat-Ko J, Burton JP, Silverman MS. 2017; Extended screening costs associated with selecting donors for fecal microbiota transplantation for treatment of metabolic syndrome-associated diseases. Open Forum Infect Dis. 4:ofx243. DOI: 10.1093/ofid/ofx243. PMID: 29255739. PMCID: PMC5730934.
Article7. Kassam Z, Dubois N, Ramakrishna B, Ling K, Qazi T, Smith M, et al. 2019; Donor screening for fecal microbiota transplantation. N Engl J Med. 381:2070–2. DOI: 10.1056/NEJMc1913670. PMID: 31665572.
Article8. Rode AA, Bytzer P, Pedersen OB, Engberg J. 2019; Establishing a donor stool bank for faecal microbiota transplantation: methods and feasibility. Eur J Clin Microbiol Infect Dis. 38:1837–47. DOI: 10.1007/s10096-019-03615-x. PMID: 31273647.
Article9. Terveer EM, van Beurden YH, Goorhuis A, Seegers JFML, Bauer MP, van Nood E, et al. 2017; How to: establish and run a stool bank. Clin Microbiol Infect. 23:924–30. DOI: 10.1016/j.cmi.2017.05.015. PMID: 28529025.
Article10. Woodworth MH, Carpentieri C, Sitchenko KL, Kraft CS. 2017; Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes. 8:225–37. DOI: 10.1080/19490976.2017.1286006. PMID: 28129018. PMCID: PMC5479407.
Article11. U.S. Food and Drug Administration. Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi-drug resistant organisms. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse. Updated on June 2019.12. Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, et al. 2019; International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 68:2111–21. DOI: 10.1136/gutjnl-2019-319548. PMID: 31563878. PMCID: PMC6872442.
Article13. Gweon TG, Lee KJ, Kang DH, Park SS, Kim KH, Seong HJ, et al. 2015; A case of toxic megacolon caused by Clostridium difficile infection and treated with fecal microbiota transplantation. Gut Liver. 9:247–50. DOI: 10.5009/gnl14152. PMID: 25721003. PMCID: PMC4351033.14. Yoon YK, Suh JW, Kang EJ, Kim JY. 2019; Efficacy and safety of fecal microbiota transplantation for decolonization of intestinal multidrug-resistant microorganism carriage: beyond Clostridioides difficile infection. Ann Med. 51:379–89. DOI: 10.1080/07853890.2019.1662477. PMID: 31468999.15. Chung KS, Ko WY. 2016. Transfusion guideline. 4th ed. Division of human blood safety surveillance, Korea Centers for Disease Control & Prevention;Cheongju: p. 3–5.16. DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. 2019; Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 381:2043–50. DOI: 10.1056/NEJMoa1910437. PMID: 31665575.17. van Beurden YH, de Groot PF, van Nood E, Nieuwdorp M, Keller JJ, Goorhuis A. 2017; Complications, effectiveness, and long term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile infection. United European Gastroenterol J. 5:868–79. DOI: 10.1177/2050640616678099. PMID: 29026601. PMCID: PMC5625865.18. Šeligová B, Lukáč Ľ, Bábelová M, Vávrová S, Sulo P. 2020; Diagnostic reliability of nested PCR depends on the primer design and threshold abundance of Helicobacter pylori in biopsy, stool, and saliva samples. Helicobacter. 25:e12680. DOI: 10.1111/hel.12680. PMID: 32057175.
Article19. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. 2017; Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 153:420–9. DOI: 10.1053/j.gastro.2017.04.022. PMID: 28456631.20. Sung H, Kim MN, Yong D, Lee M, Lee J, Lee MK, et al. 2018; Multicenter study on the association of positive Helicobacter pylori stool antigen to anemia in children. Ann Clin Microbiol. 21:58–63. DOI: 10.5145/ACM.2018.21.3.58.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening for potential infections in fecal microbiota transplantation guidelines and an experience of microbiota bank operation in Korea: a narrative review
- Fecal Microbiota Transplantation and the Brain Microbiota in Neurological Diseases
- Donor Screening for Fecal Microbiota Transplantation
- The practice of fecal microbiota transplantation in inflammatory bowel disease
- Fecal Microbiota Transplantation: Is It Safe?