Intest Res.
2024 Jan;22(1):44-64. 10.5217/ir.2023.00085.
The practice of fecal microbiota transplantation in inflammatory bowel disease
- Affiliations
-
- 1Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi, India
- KMID: 2551278
- DOI: http://doi.org/10.5217/ir.2023.00085
Abstract
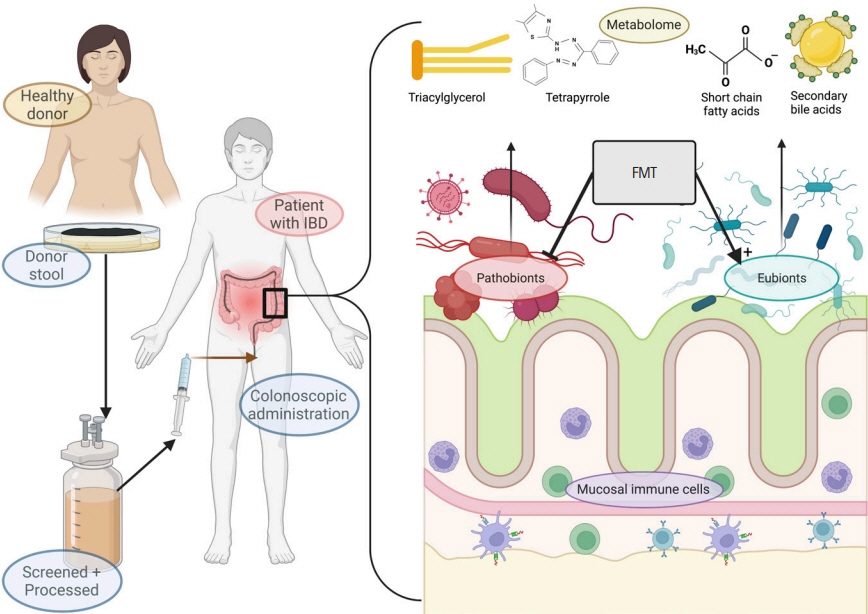
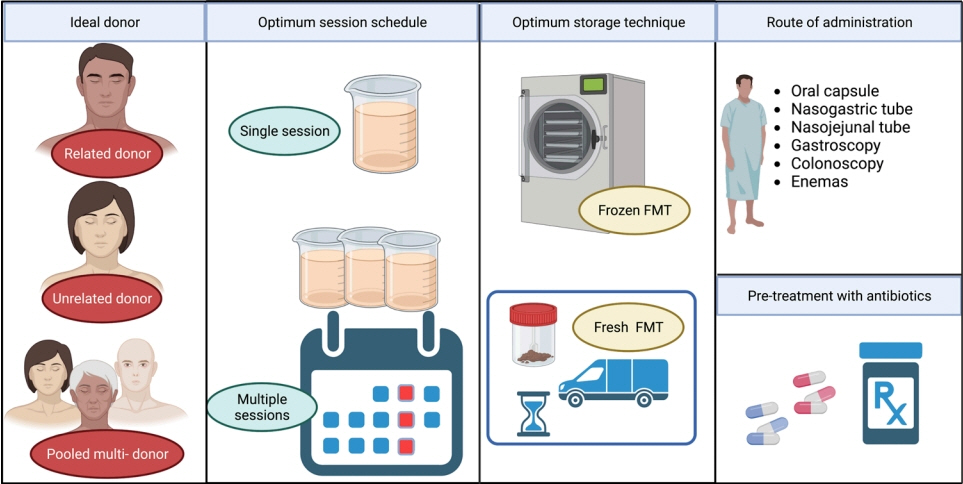
- Current evidence posits a central role for gut microbiota and the metabolome in the pathogenesis and progression of inflammatory bowel disease (IBD). Fecal microbiota transplantation (FMT) has been established as a means to manipulate this microbiome safely and sustainably. Several aspects of the technical improvement including pretreatment with antibiotics, use of frozen stool samples as well as short donor-to-recipient time are proposed to improve its response rates. Its efficacy in ulcerative colitis has been proven in clinical trials while data is emerging for Crohn’s disease. This review describes briefly the biology behind FMT, the available evidence for its use in IBD, and the host, recipient and procedural factors which determine the clinical outcomes.
Keyword
Figure
Reference
-
1. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019; 4:293–305.
Article2. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014; 63:1275–1283.
Article3. Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018; 67:108–119.
Article4. Verma R, Verma AK, Ahuja V, Paul J. Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India. J Clin Microbiol. 2010; 48:4279–4282.
Article5. Ahuja V. Inventory of a reservoir: friends & foes. Indian J Med Res. 2015; 142:4–6.6. Nguyen LH, Örtqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: a national casecontrol study in Sweden. Lancet Gastroenterol Hepatol. 2020; 5:986–995.
Article7. Kedia S, Rampal R, Paul J, Ahuja V. Gut microbiome diversity in acute infective and chronic inflammatory gastrointestinal diseases in North India. J Gastroenterol. 2016; 51:660–671.
Article8. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011; 106:563–573.
Article9. Naser SA, Sagramsingh SR, Naser AS, Thanigachalam S. Mycobacterium avium subspecies paratuberculosis causes Crohn’s disease in some inflammatory bowel disease patients. World J Gastroenterol. 2014; 20:7403–7415.
Article10. Selby W, Pavli P, Crotty B, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology. 2007; 132:2313–2319.
Article11. Khan IA, Pilli S, A S, et al. Prevalence and association of Mycobacterium avium subspecies paratuberculosis with disease course in patients with ulcero-constrictive ileocolonic disease. PLoS One. 2016; 11:e0152063.
Article12. Khan IA, Nayak B, Markandey M, et al. Differential prevalence of pathobionts and host gene polymorphisms in chronic inflammatory intestinal diseases: Crohn’s disease and intestinal tuberculosis. PLoS One. 2021; 16:e0256098.
Article13. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008; 105:16731–16736.
Article14. Magnusson MK, Strid H, Isaksson S, Simrén M, Öhman L. The mucosal antibacterial response profile and fecal microbiota composition are linked to the disease course in patients with newly diagnosed ulcerative colitis. Inflamm Bowel Dis. 2017; 23:956–966.
Article15. Machiels K, Sabino J, Vandermosten L, et al. Specific members of the predominant gut microbiota predict pouchitis following colectomy and IPAA in UC. Gut. 2017; 66:79–88.
Article16. Zhou Y, Xu ZZ, He Y, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. 2018; 3:e00188. –17.
Article17. Magnusson MK, Strid H, Sapnara M, et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis. 2016; 10:943–952.
Article18. Ananthakrishnan AN, Luo C, Yajnik V, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017; 21:603–610.
Article19. Zhou Y, He Y, Liu L, et al. Alterations in gut microbial communities across anatomical locations in inflammatory bowel diseases. Front Nutr. 2021; 8:615064.
Article20. Kim S, Kim JH, Park BO, Kwak YS. Perspectives on the therapeutic potential of short-chain fatty acid receptors. BMB Rep. 2014; 47:173–178.
Article21. Dorrestein PC, Mazmanian SK, Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity. 2014; 40:824–832.
Article22. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013; 341:569–573.
Article23. Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016; 22:598–605.
Article24. Vich Vila A, Hu S, Andreu-Sánchez S, et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut. 2023; 72:1472–1485.
Article25. Schoch CL, Seifert KA, Huhndorf S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A. 2012; 109:6241–6246.26. Hoarau G, Mukherjee PK, Gower-Rousseau C, et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. mBio. 2016; 7:e01250-16. –16.
Article27. Hager CL, Isham N, Schrom KP, et al. Effects of a novel probiotic combination on pathogenic bacterial-fungal polymicrobial biofilms. mBio. 2019; 10:e00338-19.
Article28. Imai T, Inoue R, Kawada Y, et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2019; 54:149–159.
Article29. Jain U, Ver Heul AM, Xiong S, et al. Debaryomyces is enriched in Crohn’s disease intestinal tissue and impairs healing in mice. Science. 2021; 371:1154–1159.
Article30. Limon JJ, Tang J, Li D, et al. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe. 2019; 25:377–388.
Article31. Stockdale SR, Shkoporov AN, Khokhlova EV, et al. Interpersonal variability of the human gut virome confounds disease signal detection in IBD. Commun Biol. 2023; 6:221.
Article32. Norman JM, Handley SA, Baldridge MT, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015; 160:447–460.
Article33. Yan A, Butcher J, Schramm L, Mack DR, Stintzi A. Multiomic spatial analysis reveals a distinct mucosa-associated virome. Gut Microbes. 2023; 15:2177488.
Article34. Markandey M, Bajaj A, Ilott NE, et al. Gut microbiota: sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes. 2021; 13:1990827.
Article35. Altomare A, Putignani L, Del Chierico F, et al. Gut mucosalassociated microbiota better discloses inflammatory bowel disease differential patterns than faecal microbiota. Dig Liver Dis. 2019; 51:648–656.
Article36. Kabeerdoss J, Jayakanthan P, Pugazhendhi S, Ramakrishna BS. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J Med Res. 2015; 142:23–32.
Article37. Lennon G, Balfe Á, Bambury N, et al. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Colorectal Dis. 2014; 16:–O161-O169.
Article38. Sokol H, Vasquez N, Hoyeau-Idrissi N, et al. Crypt abscess-associated microbiota in inflammatory bowel disease and acute self-limited colitis. World J Gastroenterol. 2010; 16:583587.
Article39. Markandey M, Bajaj A, Verma M, et al. Fecal microbiota transplantation refurbishes the crypt-associated microbiota in ulcerative colitis. iScience. 2023; 26:106738.
Article40. Abdelbary MM, Hatting M, Bott A, et al. The oral-gut axis: salivary and fecal microbiome dysbiosis in patients with inflammatory bowel disease. Front Cell Infect Microbiol. 2022; 12:1010853.
Article41. Atarashi K, Suda W, Luo C, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017; 358:359–365.
Article42. Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018; 24:5–14.
Article43. Paramsothy S, Nielsen S, Kamm MA, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019; 156:1440–1454.
Article44. Mocanu V, Rajaruban S, Dang J, Kung JY, Deehan EC, Madsen KL. Repeated fecal microbial transplantations and antibiotic pre-treatment are linked to improved clinical response and remission in inflammatory bowel disease: a systematic review and pooled proportion meta-analysis. J Clin Med. 2021; 10:959.
Article45. Lima SF, Gogokhia L, Viladomiu M, et al. Transferable immunoglobulin A-coated Odoribacter splanchnicus in responders to fecal microbiota transplantation for ulcerative colitis limits colonic inflammation. Gastroenterology. 2022; 162:166178.
Article46. Hoelz H, Heetmeyer J, Tsakmaklis A, et al. Is autologous fecal microbiota transfer after exclusive enteral nutrition in pediatric Crohn’s disease patients rational and feasible? Data from a feasibility test. Nutrients. 2023; 15:1742.47. Ponce-Alonso M, Garcia-Fernandez S, Aguilera L, et al. P782 A new compatibility test for donor selection for faecal microbiota transplantation in ulcerative colitis. J Crohns Colitis. 2017; 11(suppl_1):S480–S481.
Article48. U.S. Food and Drug Administration. Information pertaining to additional safety protections regarding use of fecal microbiota for transplantation: testing of stool donors for enteropathogenic Escherichia coli and Shigatoxin-producing Escherichia coli [Internet]. c2020 [cited 2023 Jul 9]. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/information-pertaining-additional-safety-protectionsregarding-use-fecal-microbiota-transplantation-0.49. Ianiro G, Mullish BH, Kelly CR, et al. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020; 5:430–432.
Article50. Bénard MV, de Bruijn CM, Fenneman AC, et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS One. 2022; 17:e0276323.
Article51. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017; 66:569–580.
Article52. Lopetuso LR, Deleu S, Godny L, et al. The first international Rome consensus conference on gut microbiota and faecal microbiota transplantation in inflammatory bowel disease. Gut. 2023; 72:1642–1650.
Article53. Bilinski J, Dziurzynski M, Grzesiowski P, et al. Fresh versus frozen stool for fecal microbiota transplantation-assessment by multimethod approach combining culturing, flow cytometry, and next-generation sequencing. Front Microbiol. 2022; 13:872735.
Article54. Gangwani MK, Aziz M, Aziz A, et al. Fresh versus frozen versus lyophilized fecal microbiota transplant for recurrent Clostridium difficile infection: a systematic review and network meta-analysis. J Clin Gastroenterol. 2023; 57:239–245.
Article55. Zhao HL, Chen SZ, Xu HM, et al. Efficacy and safety of fecal microbiota transplantation for treating patients with ulcerative colitis: a systematic review and meta-analysis. J Dig Dis. 2020; 21:534–548.
Article56. Zhang T, Lu G, Zhao Z, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020; 11:251–266.
Article57. Ding X, Li Q, Li P, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019; 42:869–880.
Article58. Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017; 152:799–811.
Article59. Stallmach A, Grunert P, Stallhofer J, et al. Transfer of FRozen Encapsulated multi-donor Stool filtrate for active ulcerative Colitis (FRESCO): study protocol for a prospective, multicenter, double-blind, randomized, controlled trial. Trials. 2022; 23:173.
Article60. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019; 321:156–164.
Article61. Shimizu H, Arai K, Asahara T, et al. Stool preparation under anaerobic conditions contributes to retention of obligate anaerobes: potential improvement for fecal microbiota transplantation. BMC Microbiol. 2021; 21:275.
Article62. Chu ND, Smith MB, Perrotta AR, Kassam Z, Alm EJ. Profiling living bacteria informs preparation of fecal microbiota transplantations. PLoS One. 2017; 12:e0170922.
Article63. Papanicolas LE, Choo JM, Wang Y, et al. Bacterial viability in faecal transplants: which bacteria survive? EBioMedicine. 2019; 41:509–516.
Article64. Singh A, Mahajan R, Kahlon BK, et al. Early fecal microbiome transfer after donor defecation determines response in patients with moderate to severe ulcerative colitis. Indian J Gastroenterol. 2022; 41:389–396.
Article65. Zhang YJ, Bousvaros A, Docktor M, et al. Higher alpha diversity and Lactobacillus blooms are associated with better engraftment after fecal microbiota transplant in inflammatory bowel disease. c2023; [cited 2023 Jul 19]. https://doi.org/10.1101/2023.01.30.23285033.
Article66. Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004; 126:1620–1633.
Article67. Ji SK, Yan H, Jiang T, et al. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front Microbiol. 2017; 8:1208.
Article68. Ishikawa D, Sasaki T, Osada T, et al. Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflamm Bowel Dis. 2017; 23:116–125.
Article69. Kump P, Wurm P, Gröchenig HP, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol Ther. 2018; 47:67–77.
Article70. Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018; 67:19201941.
Article71. Gweon TG, Lee YJ, Kim KO, et al. Clinical practice guidelines for fecal microbiota transplantation in Korea. J Neurogastroenterol Motil. 2022; 28:28–42.
Article72. Jalanka J, Salonen A, Salojärvi J, et al. Effects of bowel cleansing on the intestinal microbiota. Gut. 2015; 64:1562–1568.
Article73. O’Brien CL, Allison GE, Grimpen F, Pavli P. Impact of colonoscopy bowel preparation on intestinal microbiota. PLoS One. 2013; 8:e62815.
Article74. Harrell L, Wang Y, Antonopoulos D, et al. Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One. 2012; 7:e32545.
Article75. Tariq R, Hayat M, Pardi D, Khanna S. Predictors of failure after fecal microbiota transplantation for recurrent Clostridioides difficile infection: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2021; 40:1383–1392.
Article76. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, openlabel, controlled pilot study. Clin Infect Dis. 2014; 58:1515–1522.
Article77. Yang Z, Bu C, Yuan W, et al. Fecal microbiota transplant via endoscopic delivering through small intestine and colon: no difference for Crohn’s disease. Dig Dis Sci. 2020; 65:150–157.
Article78. Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: a systematic review and meta-analysis. Biomed Res Int. 2018; 2018:8941340.
Article79. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110118.
Article80. Haifer C, Paramsothy S, Kaakoush NO, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2022; 7:141–151.
Article81. Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017; 318:1985–1993.
Article82. Vaughn BP, Fischer M, Kelly CR, et al. Effectiveness and safety of colonic and capsule fecal microbiota transplantation for recurrent Clostridioides difficile infection. Clin Gastroenterol Hepatol. 2023; 21:1330–1337.
Article83. Wei ZJ, Dong HB, Ren YT, Jiang B. Efficacy and safety of fecal microbiota transplantation for the induction of remission in active ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials. Ann Transl Med. 2022; 10:802.
Article84. El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020; 69:859–867.
Article85. Ren R, Gao X, Shi Y, et al. Long-term efficacy of low-intensity single donor fecal microbiota transplantation in ulcerative colitis and outcome-specific gut bacteria. Front Microbiol. 2021; 12:742255.
Article86. Baunwall SM, Lee MM, Eriksen MK, et al. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine. 2020; 29-30:100642.
Article87. El-Salhy M, Hausken T, Hatlebakk JG. Increasing the dose and/or repeating faecal microbiota transplantation (FMT) increases the response in patients with irritable bowel syndrome (IBS). Nutrients. 2019; 11:1415.
Article88. Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003; 37:42–47.
Article89. Chen M, Liu XL, Zhang YJ, Nie YZ, Wu KC, Shi YQ. Efficacy and safety of fecal microbiota transplantation by washed preparation in patients with moderate to severely active ulcerative colitis. J Dig Dis. 2020; 21:621–628.
Article90. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.
Article91. Kedia S, Virmani S, K Vuyyuru S, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 2022; 71:24012413.
Article92. Sood A, Singh A, Mahajan R, et al. Clinical predictors of response to faecal microbiota transplantation in patients with active ulcerative colitis. J Crohns Colitis. 2020; 15:238–243.
Article93. Kazerouni A, Wein LM. Exploring the efficacy of pooled stools in fecal microbiota transplantation for microbiota-associated chronic diseases. PLoS One. 2017; 12:e0163956.
Article94. Levast B, Fontaine M, Nancey S, Dechelotte P, Doré J, Lehert P. Single-donor and pooling strategies for fecal microbiota transfer product preparation in ulcerative colitis: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2023; 14:e00568.
Article95. Li H, Li Y, Qian J. What is the “optimal formula” for donor selection and feces processing for fecal microbiota transplantation in ulcerative colitis? Chin Med J (Engl). 2023; 136:14101412.
Article96. Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The superdonor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. 2019; 9:2.
Article97. Okahara K, Ishikawa D, Nomura K, et al. Matching between donors and ulcerative colitis patients is important for longterm maintenance after fecal microbiota transplantation. J Clin Med. 2020; 9:1650.
Article98. Barnes D, Ng K, Smits S, Sonnenburg J, Kassam Z, Park KT. Competitively selected donor fecal microbiota transplantation: butyrate concentration and diversity as measures of donor quality. J Pediatr Gastroenterol Nutr. 2018; 67:185–187.99. Haifer C, Luu LD, Paramsothy S, Borody TJ, Leong RW, Kaakoush NO. Microbial determinants of effective donors in faecal microbiota transplantation for UC. Gut. 2023; 72:92–100.
Article100. He R, Li P, Wang J, Cui B, Zhang F, Zhao F. The interplay of gut microbiota between donors and recipients determines the efficacy of fecal microbiota transplantation. Gut Microbes. 2022; 14:2100197.
Article101. Olesen SW, Gerardin Y. Re-evaluating the evidence for faecal microbiota transplantation ‘super-donors’ in inflammatory bowel disease. J Crohns Colitis. 2021; 15:453–461.
Article102. Rees NP, Shaheen W, Quince C, et al. Systematic review of donor and recipient predictive biomarkers of response to faecal microbiota transplantation in patients with ulcerative colitis. EBioMedicine. 2022; 81:104088.
Article103. Zhang B, Yang L, Ning H, et al. A matching strategy to guide donor selection for ulcerative colitis in fecal microbiota transplantation: meta-analysis and analytic hierarchy process. Microbiol Spectr. 2023; 11:e0215921.
Article104. Bajaj A, Markandey M, Singh M, et al. P702 Faecal microbiota transplantation sculpts the faecal and mucosal microbial and metabolomic profiles in patients with ulcerative colitis. J Crohns Colitis. 2022; 16(Suppl_1):i598–i601.
Article105. Tkach S, Dorofeyev A, Kuzenko I, et al. Efficacy and safety of fecal microbiota transplantation via colonoscopy as add-on therapy in patients with mild-to-moderate ulcerative colitis: a randomized clinical trial. Front Med (Lausanne). 2023; 9:1049849.
Article106. Sarbagili Shabat C, Scaldaferri F, Zittan E, et al. Use of faecal transplantation with a novel diet for mild to moderate active ulcerative colitis: the CRAFT UC randomised controlled trial. J Crohns Colitis. 2022; 16:369–378.
Article107. Pai N, Popov J, Hill L, et al. Results of the first pilot randomized controlled trial of fecal microbiota transplant in pediatric ulcerative colitis: lessons, limitations, and future prospects. Gastroenterology. 2021; 161:388–393.
Article108. Crothers JW, Chu ND, Nguyen LT, et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021; 21:281.
Article109. Fang H, Fu L, Li X, et al. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb Cell Fact. 2021; 20:18.
Article110. Březina J, Bajer L, Wohl P, et al. Fecal microbial transplantation versus mesalamine enema for treatment of active leftsided ulcerative colitis: results of a randomized controlled trial. J Clin Med. 2021; 10:2753.
Article111. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017; 389:1218–1228.
Article112. Sood A, Mahajan R, Singh A, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. 2019; 13:1311–1317.
Article113. Lahtinen P, Jalanka J, Mattila E, et al. Fecal microbiota transplantation for the maintenance of remission in patients with ulcerative colitis: a randomized controlled trial. World J Gastroenterol. 2023; 29:2666–2678.
Article114. El Hage Chehade N, Ghoneim S, Shah S, et al. Efficacy of fecal microbiota transplantation in the treatment of active ulcerative colitis: a systematic review and meta-analysis of double-blind randomized controlled trials. Inflamm Bowel Dis. 2023; 29:808–817.
Article115. Imdad A, Pandit NG, Zaman M, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2023; 4–CD012774.
Article116. Vuyyuru SK, Kedia S, Kalaivani M, et al. Efficacy and safety of fecal transplantation versus targeted therapies in ulcerative colitis: network meta-analysis. Future Microbiol. 2021; 16:12151227.
Article117. Haifer C, Saikal A, Paramsothy R, et al. Response to faecal microbiota transplantation in ulcerative colitis is not sustained long term following induction therapy. Gut. 2021; 70:22102211.
Article118. Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015; 13:298.
Article119. Sood A, Mahajan R, Juyal G, et al. Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: a real world intention-to-treat analysis. Intest Res. 2019; 17:78–86.
Article120. Seth AK, Jain P. Fecal microbiota transplantation for induction of remission, maintenance and rescue in patients with corticosteroid-dependent ulcerative colitis: a long-term follow-up real-world cohort study. Intest Res. 2022; 20:251–259.
Article121. Jadhav A, Bajaj A, Xiao Y, Markandey M, Ahuja V, Kashyap PC. Role of diet-microbiome interaction in gastrointestinal disorders and strategies to modulate them with microbiome-targeted therapies. Annu Rev Nutr. 2023; 43:355–383.
Article122. Sigall-Boneh R, Levine A, Lomer M, et al. Research gaps in diet and nutrition in inflammatory bowel disease. a topical review by D-ECCO Working Group [Dietitians of ECCO]. J Crohns Colitis. 2017; 11:1407–1419.
Article123. Costello SP, Day A, Yao CK, Bryant RV. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep. 2020; 13:e233135.
Article124. Kumagai H, Yokoyama K, Imagawa T, et al. Failure of fecal microbiota transplantation in a three-year-old child with severe refractory ulcerative colitis. Pediatr Gastroenterol Hepatol Nutr. 2016; 19:214–220.
Article125. Zhou S, Cui Y, Zhang Y, Zhao T, Cong J. Fecal microbiota transplantation for induction of remission in Crohn’s disease: a systematic review and meta-analysis. Int J Colorectal Dis. 2023; 38:62.
Article126. ClinicalTrials.gov [Internet]. U.S. National Library of Medicine, 2022 [cited 2023 Nov 10]. https://classic.clinicaltrials.gov/ct2/show/NCT03078803.127. Sokol H, Landman C, Seksik P, et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome. 2020; 8:12.
Article128. Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016; 387:156–167.129. Arora U, Kedia S, Garg P, et al. Colonic Crohn’s disease is associated with less aggressive disease course than ileal or ileocolonic disease. Dig Dis Sci. 2018; 63:1592–1599.
Article130. Haac BE, Palmateer NC, Seaton ME, VanYPeren R, Fraser CM, Bafford AC. A distinct gut microbiota exists within Crohn’s disease-related perianal fistulae. J Surg Res. 2019; 242:118–128.
Article131. Allegretti JR, Kassam Z, Carrellas M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am J Gastroenterol. 2019; 114:10711079.
Article132. Zella GC, Hait EJ, Glavan T, et al. Distinct microbiome in pouchitis compared to healthy pouches in ulcerative colitis and familial adenomatous polyposis. Inflamm Bowel Dis. 2011; 17:1092–1100.
Article133. Cold F, Kousgaard SJ, Halkjaer SI, et al. Fecal microbiota transplantation in the treatment of chronic pouchitis: a systematic review. Microorganisms. 2020; 8:1433.
Article134. Xiang L, Ding X, Li Q, et al. Efficacy of faecal microbiota transplantation in Crohn’s disease: a new target treatment? Microb Biotechnol. 2020; 13:760–769.
Article135. Chen T, Zhou Q, Zhang D, et al. Effect of faecal microbiota transplantation for treatment of Clostridium difficile infection in patients with inflammatory bowel disease: a systematic review and meta-analysis of cohort studies. J Crohns Colitis. 2018; 12:710–717.
Article136. Hirten RP, Grinspan A, Fu SC, et al. Microbial engraftment and efficacy of fecal microbiota transplant for Clostridium difficile in patients with and without inflammatory bowel disease. Inflamm Bowel Dis. 2019; 25:969–979.
Article137. Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013; 56:597–601.
Article138. Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis. 2015; 21:556563.
Article139. Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021; 53:33–42.
Article140. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019; 381:2043–2050.
Article141. Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014; 8:252–253.
Article142. Baxter M, Ahmad T, Colville A, Sheridan R. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin Infect Dis. 2015; 61:136–137.
Article143. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014; 109:10651071.
Article144. Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017; 46:479–493.
Article145. Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012; 142:490–496.
Article146. Merrick B, Allen L, Zain NMM, Forbes B, Shawcross DL, Goldenberg SD. Regulation, risk and safety of faecal microbiota transplant. Infect Prev Pract. 2020; 2:100069.
Article147. Porcari S, Benech N, Valles-Colomer M, et al. Key determinants of success in fecal microbiota transplantation: from microbiome to clinic. Cell Host Microbe. 2023; 31:712–733.
Article148. U.S. Food and Drug Administration. Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies [Internet]. c2022 [cited 2023 Jul 9]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcementpolicy-regarding-investigational-new-drug-requirementsuse-fecal-microbiota.149. U.S. Food and Drug Administration. FDA approves first fecal microbiota product [Internet]. c2022 [cited 2023 May 8]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product.150. European Commission. Proposal for a Regulation on substances of human origin [Internet]. c2023 [cited 2023 Jul 10]. https://health.ec.europa.eu/blood-tissues-cells-and-organs/overview/proposal-regulation-substances-human-origin_en.151. de Stefano MC, Mazzanti B, Vespasiano F, Lombardini L, Cardillo M. The regulatory approach for faecal microbiota transplantation as treatment for Clostridioides difficile infection in Italy. Antibiotics (Basel). 2022; 11:480.
Article152. Medicines and Healthcare products Regulatory Agency. A guide to what is a medicinal product [Internet]. c2020 [cited 2023 Jul 10]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/872742/GN8_FINAL_10_03_2020__combined_.pdf.153. U.S. Food and Drug Administration Center for Biologics Evaluation and Research. Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information [Internet]. c2021 [cited 2023 Sep 9]. https://www.fda.gov/media/82945/download.154. Lee C, Louie T, Bancke L, et al. Safety of fecal microbiota, livejslm (REBYOTA™) in individuals with recurrent Clostridioides difficile infection: data from five prospective clinical trials. Therap Adv Gastroenterol. 2023; 16:17562848231174277.155. Henn MR, O’Brien EJ, Diao L, et al. A phase 1b safety study of SER-287, a spore-based microbiome therapeutic, for active mild to moderate ulcerative colitis. Gastroenterology. 2021; 160:115–127.
Article156. Businesswire. Seres therapeutics announces topline results for SER-287 phase 2b study in mild-to-moderate ulcerative colitis [Internet]. c2021 [cited 2023 Jul 18]. https://www.businesswire.com/news/home/20210722005276/en/Seres-Therapeutics-Announces-Topline-Results-for-SER-287-Phase-2bStudy-in-Mild-to-Moderate-Ulcerative-Colitis.157. Oka A, Sartor RB. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig Dis Sci. 2020; 65:757–788.
Article158. Peng Z, Xiang J, He Z, et al. Colonic transendoscopic enteral tubing: a novel way of transplanting fecal microbiota. Endosc Int Open. 2016; 4:E610–E613.
Article159. Ianiro G, Punčochář M, Karcher N, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. 2022; 28:1913–1923.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is there a potential role of fecal microbiota transplantation in the treatment of inflammatory bowel disease?
- Efficacy and Safety of Fecal Microbiota Transplantation and Prospect of Microbe-based Therapies for Inflammatory Bowel Disease
- Microbial Modulation in Inflammatory Bowel Diseases
- Fecal Microbiota Transplantation and the Brain Microbiota in Neurological Diseases
- Fecal Microbiota Transplantation beyond Clostridioides Difficile Infection