Ann Clin Microbiol.
2024 Mar;27(1):11-17. 10.5145/ACM.2024.27.1.3.
Screening for potential infections in fecal microbiota transplantation guidelines and an experience of microbiota bank operation in Korea: a narrative review
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2556214
- DOI: http://doi.org/10.5145/ACM.2024.27.1.3
Abstract
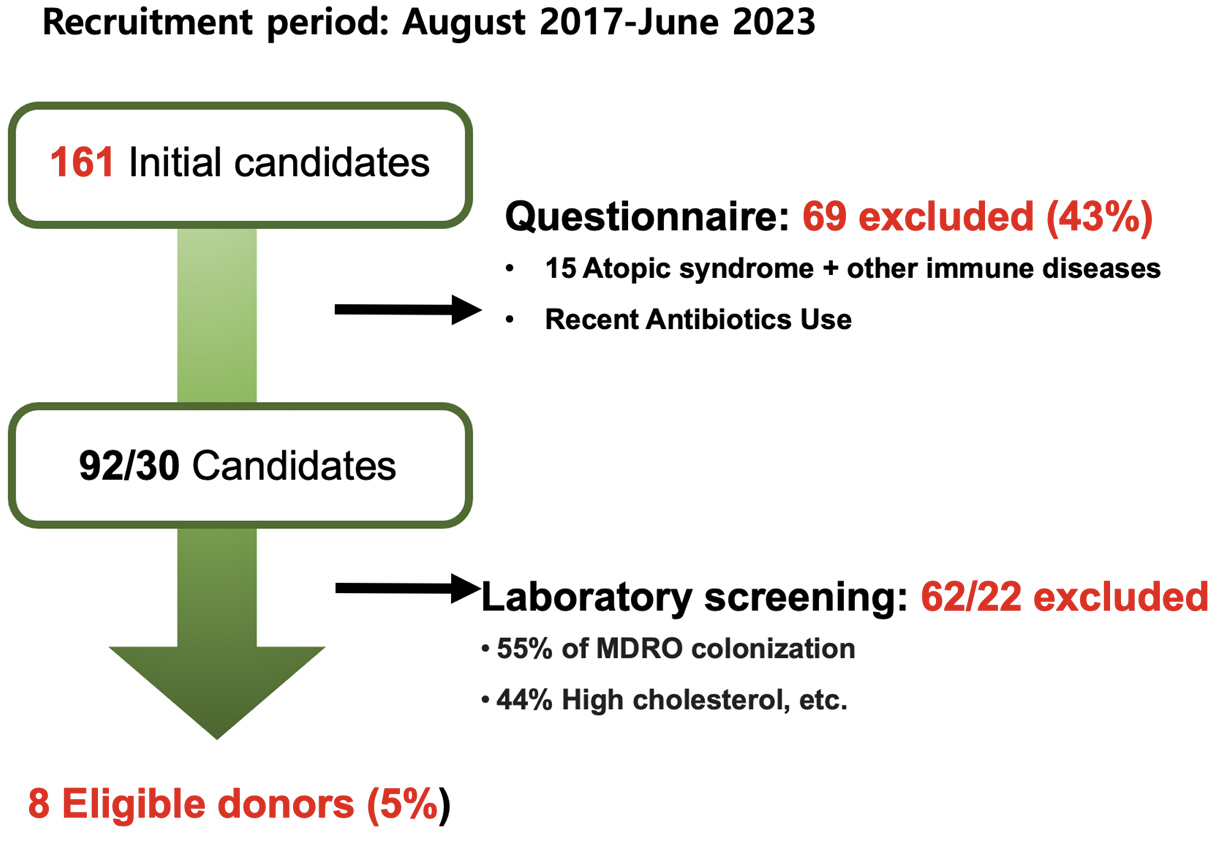
- Fecal microbiota transplantation (FMT) involves the transfer of fecal microbiota from healthy donors to patients to rectify dysbiosis and restore the functionality of the gut microbiota to a healthy state. Donor selection is important to minimize the risk of FMT. Donor selection for FMT is primarily focused on screening for potential infections. A complete consensus on screening tests and checkpoints is lacking and controversial; nevertheless, most guidelines agree to rule out certain infections, such as syphilis; hepatitis A, B, and C; and HIV. In most guidelines, stool testing includes testing for Clostridioides difficile and other enteric pathogens. The Korean FMT guidelines for C. difficile infections were published in 2022. The guidelines recommend serological and stool testing for donor candidates, with recommendations for stool testing providing targets for screening using specific test methods. Donor screening by Microbiotix Inc., a fecal microbiota bank in Korea, between 2017 and 2023 showed that only 5% of potential FMT donors were eligible for repeat donation. The future of FMT remains uncertain, with possibilities ranging from continuation to restrictions, and the development of synthetic microbiota preparations. Legislative support is crucial for advancing this field and providing hope and a potential cure for previously incurable patients.
Figure
Reference
-
1. Qu Z, Tian P, Yang B, Zhao J, Wang G, Chen W. Fecal microbiota transplantation for diseases: therapeutic potential, methodology, risk management in clinical practice. Life Sci 2022;304:120719.2. Waller KMJ, Leong RW, Paramsothy S. An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases. J Gastroenterol Hepatol 2022;37:246-55.3. Biazzo M and Deidda G. Fecal microbiota transplantation as new therapeutic avenue for human diseases. J Clin Med 2022;11:4119.4. Moon CM and Hong SN. Fecal microbiota transplantation beyond Clostridioides difficile infection. Clin Endosc 2021;54:149-51.5. Kang GU, Park S, Jung Y, Jee JJ, Kim MS, Lee S, et al. Exploration of potential gut microbiota-derived biomarkers to predict the success of fecal microbiota transplantation in ulcerative colitis: a prospective cohort in Korea. Gut Liver 2022;16:775-85.6. Park JH. Clinical usefulness of fecal microbiota transplantation. J Neurogastroenterol Motil 2017;23:149-50.7. Gweon TG, Lee YJ, Yim SK, Kim SY, Choi CH, Cho YS, et al. Recognition and attitudes of Korean physicians toward fecal microbiota transplantation: a survey study. Korean J Intern Med 2023;38:48-55.8. Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017;66:569-80.9. Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019;68:2111-21.10. Gweon TG, Lee YJ, Kim KO, Yim SK, Soh JS, Kim SY, et al. Clinical practice guidelines for fecal microbiota transplantation in Korea. J Neurogastroenterol Motil 2022;28:28-42.11. Barnes D and Park KT. Donor considerations in fecal microbiota transplantation. Curr Gastroenterol Rep 2017;19:10.12. Food and Drug Administration. Fecal microbiota fortransplantation: safety alert - risk of serious adverse events likely due to transmission of pathogenic organisms. https://www.fda.gov/safety/ medical-product-safety-information/fecal-microbiota-transplantationsafety-alert-risk-seriousadverse-events-likely-due-transmission [Online] (last visited on 26 February 2024).13. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020;158:1831-3 e3.14. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020;323:1843-4.15. Woodworth MH, Neish EM, Miller NS, Dhere T, Burd EM, Carpentieri C, et al. Laboratory testing of donors and stool samples for fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Microbiol 2017;55:1002-10.16. Seong H, Lee SK, Cheon JH, Yong DE, Koh H, Kang YK, et al. Fecal microbiota transplantation for multidrug-resistant organism: efficacy and response prediction. J Infect 2020;81:719-25.17. Lee JJ, Yong D, Suk KT, Kim DJ, Woo HJ, Lee SS, et al. Alteration of gut microbiota in carbapenem-resistant Enterobacteriaceae carriers during fecal microbiota transplantation according to decolonization periods. Microorganisms 2021;9:352.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fecal Microbiota Transplantation and the Brain Microbiota in Neurological Diseases
- Donor Screening for Fecal Microbiota Transplantation
- Safety and effectiveness of fecal microbiota transplantation: a systematic review
- Fecal Microbiota Transplantation: Is It Safe?
- Refractory Clostridium difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient