Anesth Pain Med.
2021 Jan;16(1):60-67. 10.17085/apm.20056.
Comparison of postoperative pulmonary complications between sugammadex and neostigmine in lung cancer patients undergoing video-assisted thoracoscopic lobectomy: a prospective double-blinded randomized trial
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- KMID: 2512283
- DOI: http://doi.org/10.17085/apm.20056
Abstract
- Background
Reversal of neuromuscular blockade (NMB) at the end of surgery is important for reducing postoperative residual NMB; this is associated with an increased risk of postoperative pulmonary complications (PPCs). Moreover, PPCs are associated with poor prognosis after video-assisted thoracoscopic surgery (VATS) for lobectomy. We compared the effects of two reversal agents, sugammadex and neostigmine, on the incidence of PPCs and duration of hospital stay in patients undergoing VATS lobectomy.
Methods
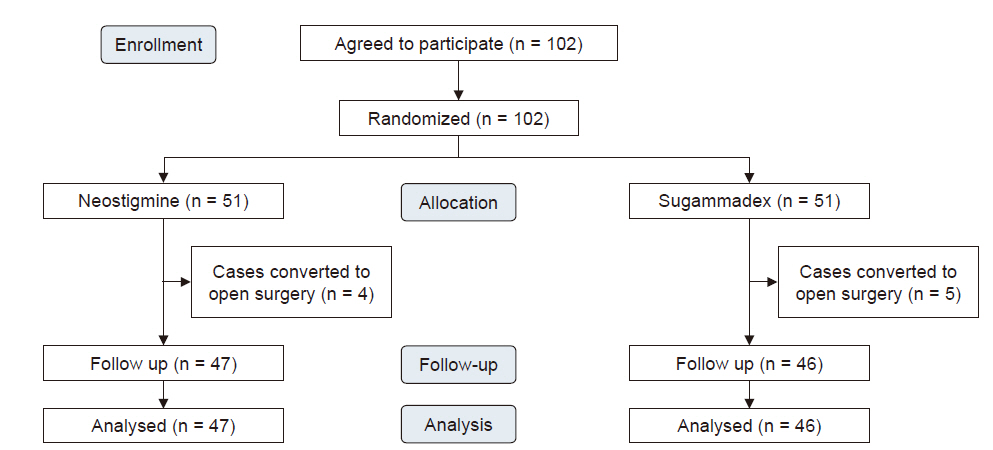
After VATS lobectomy was completed under neuromuscular monitoring, the sugammadex group (n = 46) received sugammadex 2 mg/kg, while the neostigmine group (n = 47) received neostigmine 0.05 mg/kg with atropine 0.02 mg/kg after at least the third twitch in response to the train of four stimulation. The primary outcome was incidence of PPCs. The secondary outcomes were duration of hospital stay and intensive care unit (ICU) admission.
Results
There was no significant difference in the incidence of PPCs for both the sugammadex and neostigmine groups (32.6% and 40.4%, respectively; risk difference = 0.08; 95% confidence interval = [−0.12, 0.27]; P = 0.434). The lengths of hospital (P = 0.431) and ICU (P = 0.964) stays were not significantly different between the two groups.
Conclusions
The clinical use of sugammadex and neostigmine in NMB reversal for patients undergoing VATS lobectomy was not significantly different in the incidence of PPCs and duration of hospital and ICU stay.
Keyword
Figure
Cited by 2 articles
-
Choice of neuromuscular block reversal agent to reduce postoperative pulmonary complications
Sung-Ae Cho, Tae-Yun Sung
Anesth Pain Med. 2022;17(2):121-131. doi: 10.17085/apm.22146.A message from the Editor-in-Chief and Editorial Board, 2023: journal metrics and statistics, and appreciation to reviewers
Jun Hyun Kim, Hyun Kang
Anesth Pain Med. 2024;19(1):1-4. doi: 10.17085/apm.24008.
Reference
-
1. D'Andrilli A, Rendina EA. Enhanced recovery after surgery (ERAS) and fast-track in video-assisted thoracic surgery (VATS) lobectomy: preoperative optimisation and care-plans. J Vis Surg. 2018; 4:4.2. Gao S, Barello S, Chen L, Chen C, Che G, Cai K, et al. Clinical guidelines on perioperative management strategies for enhanced recovery after lung surgery. Transl Lung Cancer Res. 2019; 8:1174–87.3. Cho HC, Lee JH, Lee SC, Park SY, Rim JC, Choi SR. Use of sugammadex in lung cancer patients undergoing video-assisted thoracoscopic lobectomy. Korean J Anesthesiol. 2017; 70:420–5.4. Schepens T, Cammu G. Neuromuscular blockade: what was, is and will be. Acta Anaesthesiol Belg. 2014; 65:151–9.5. Martinez-Ubieto J, Ortega-Lucea S, Pascual-Bellosta A, Arazo-Iglesias I, Gil-Bona J, Jimenez-Bernardó T, et al. Prospective study of residual neuromuscular block and postoperative respiratory complications in patients reversed with neostigmine versus sugammadex. Minerva Anestesiol. 2016; 82:735–42.6. Hristovska AM, Duch P, Allingstrup M, Afshari A. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2018; 73:631–41.7. Luo J, Chen S, Min S, Peng L. Reevaluation and update on efficacy and safety of neostigmine for reversal of neuromuscular blockade. Ther Clin Risk Manag. 2018; 14:2397–406.8. Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R, Espinosa A, Martínez-Hurtado E, Fernández-Pérez C, et al. Evidence Anaesthesia Review Group. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia. 2015; 70:1441–52.9. Paech MJ, Kaye R, Baber C, Nathan EA. Recovery characteristics of patients receiving either sugammadex or neostigmine and glycopyrrolate for reversal of neuromuscular block: a randomised controlled trial. Anaesthesia. 2018; 73:340–7.10. Oh TK, Ryu JH, Nam S, Oh AY. Association of neuromuscular reversal by sugammadex and neostigmine with 90-day mortality after non-cardiac surgery. BMC Anesthesiol. 2020; 20:41.11. de Menezes CC, Peceguini LA, Silva ED, Simões CM. Use of sugammadex after neostigmine incomplete reversal of rocuronium-induced neuromuscular blockade. Rev Bras Anestesiol. 2012; 62:543–7.12. Pühringer FK, Gordon M, Demeyer I, Sparr HJ, Ingimarsson J, Klarin B, et al. Sugammadex rapidly reverses moderate rocuronium- or vecuronium-induced neuromuscular block during sevoflurane anaesthesia: a dose-response relationship. Br J Anaesth. 2010; 105:610–9.13. Şentürk M, Slinger P, Cohen E. Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Pract Res Clin Anaesthesiol. 2015; 29:357–69.14. Sasaki N, Meyer MJ, Malviya SA, Stanislaus AB, MacDonald T, Doran ME, et al. Effects of neostigmine reversal of nondepolarizing neuromuscular blocking agents on postoperative respiratory outcomes: a prospective study. Anesthesiology. 2014; 121:959–68.15. Schepens T, Cammu G, Maes S, Desmedt B, Vos W, Deseure K. [Functional respiratory imaging after neostigmine- or sugammadex-enhanced recovery from neuromuscular blockade in the anesthetised rat: a randomised controlled pilot study]. Rev Bras Anestesiol. 2017; 67:443–9. Portuguese.16. Paton F, Paulden M, Chambers D, Heirs M, Duffy S, Hunter JM, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth. 2010; 105:558–67.17. Min KC, Woo T, Assaid C, McCrea J, Gurner DM, Sisk CM, et al. Incidence of hypersensitivity and anaphylaxis with sugammadex. J Clin Anesth. 2018; 47:67–73.18. An J, Kim E, Lee J, Kim H, Son J, Huh J, et al. Comparison of sugammadex and pyridostigmine bromide for reversal of rocuronium-induced neuromuscular blockade in short-term pediatric surgery: a prospective randomized study. Anesth Pain Med. 2019; 14:288–93.19. Togioka BM, Yanez D, Aziz MF, Higgins JR, Tekkali P, Treggiari MM. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br J Anaesth. 2020; 124:553–61.20. Agostini PJ, Lugg ST, Adams K, Smith T, Kalkat MS, Rajesh PB, et al. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J Cardiothorac Surg. 2018; 13:28.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of sugammadex in lung cancer patients undergoing video-assisted thoracoscopic lobectomy
- Video-assisted thoracoscopic lobectomy for lung cancer
- Management of Complications During Video-Assisted Thoracic Surgery Lung Resection and Lymph Node Dissection
- Comparison of oncological outcomes of single-port versus multi-port video-assisted thoracoscopic surgery for non-small-cell lung cancer: a propensity-matched analysis
- Robot-Assisted Thoracic Surgery in Non-small Cell Lung Cancer