J Bacteriol Virol.
2020 Dec;50(4):235-245. 10.4167/jbv.2020.50.4.235.
Effect of Photodynamic Therapy Enhanced by Methylene Blue on Drug-resistant Mycobacterium smegmatis
- Affiliations
-
- 1Department of Microbiology, Kosin University College of Medicine, Busan 49267, Republic of Korea
- 2Department of Microbiology and Immunology, Indiana University School of Medicine-Northwest, Gary, IN 46408 USA
- KMID: 2512143
- DOI: http://doi.org/10.4167/jbv.2020.50.4.235
Abstract
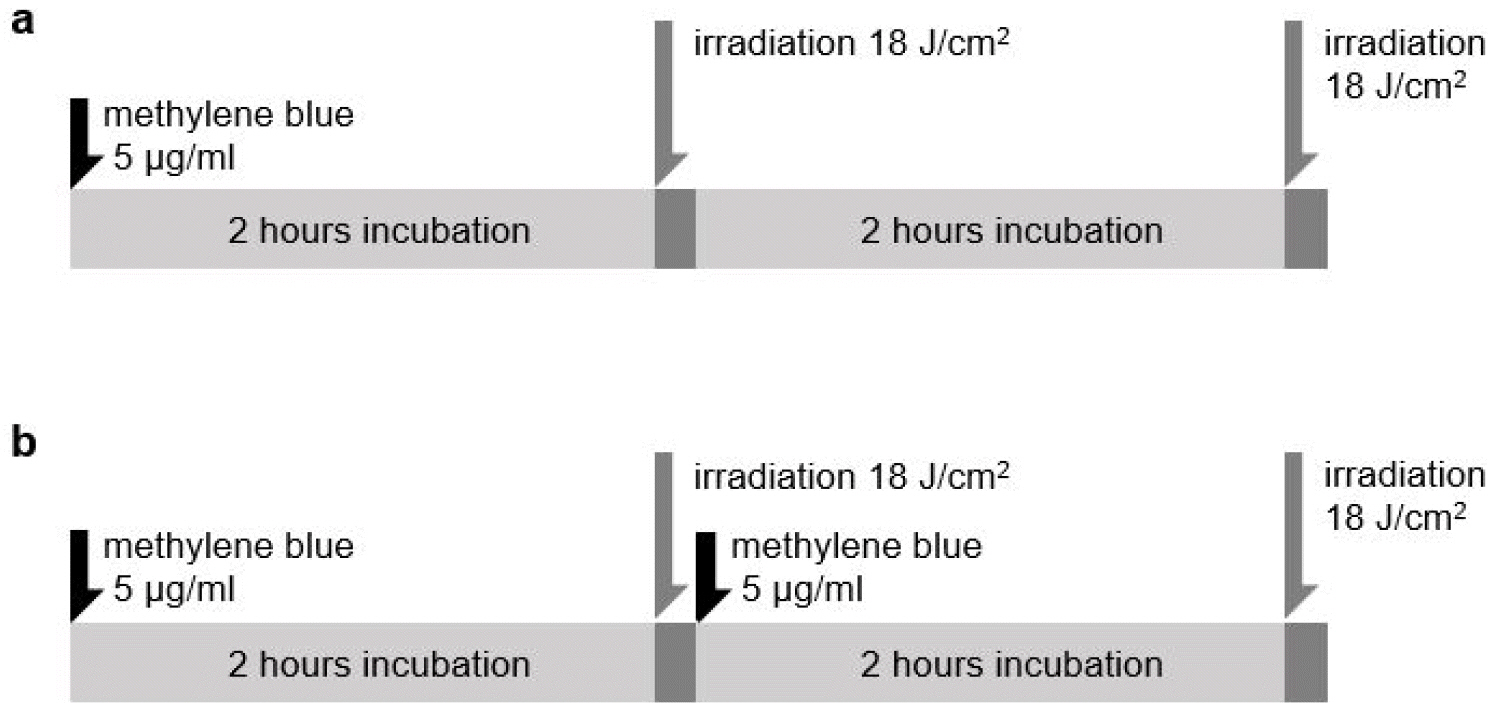
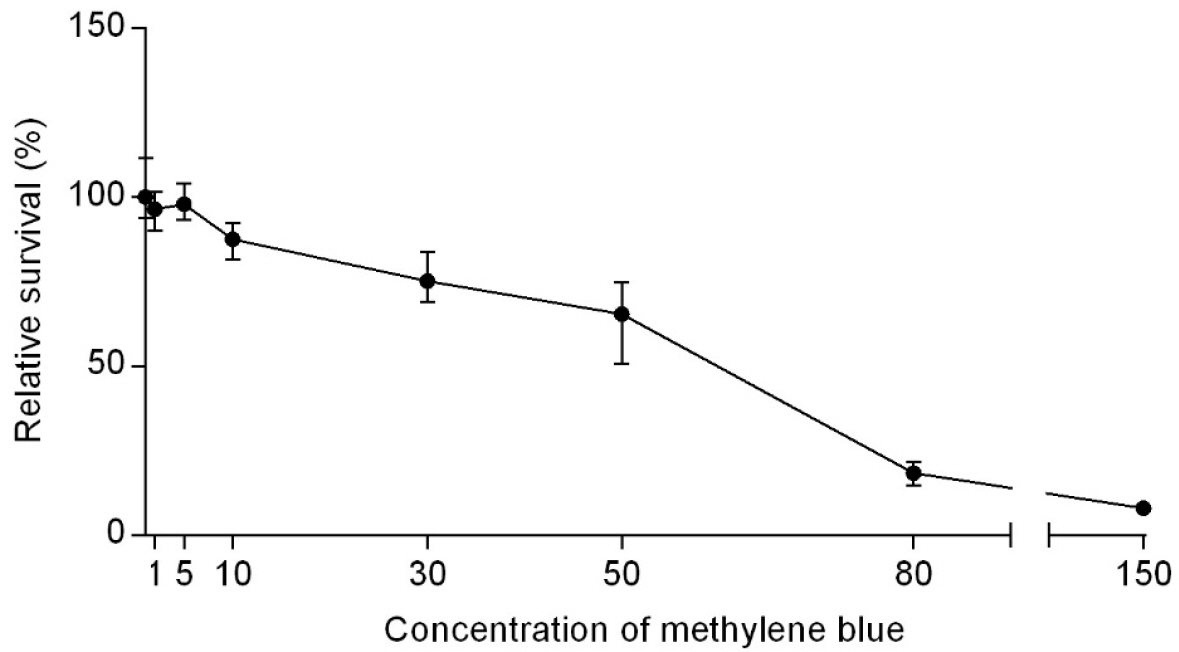
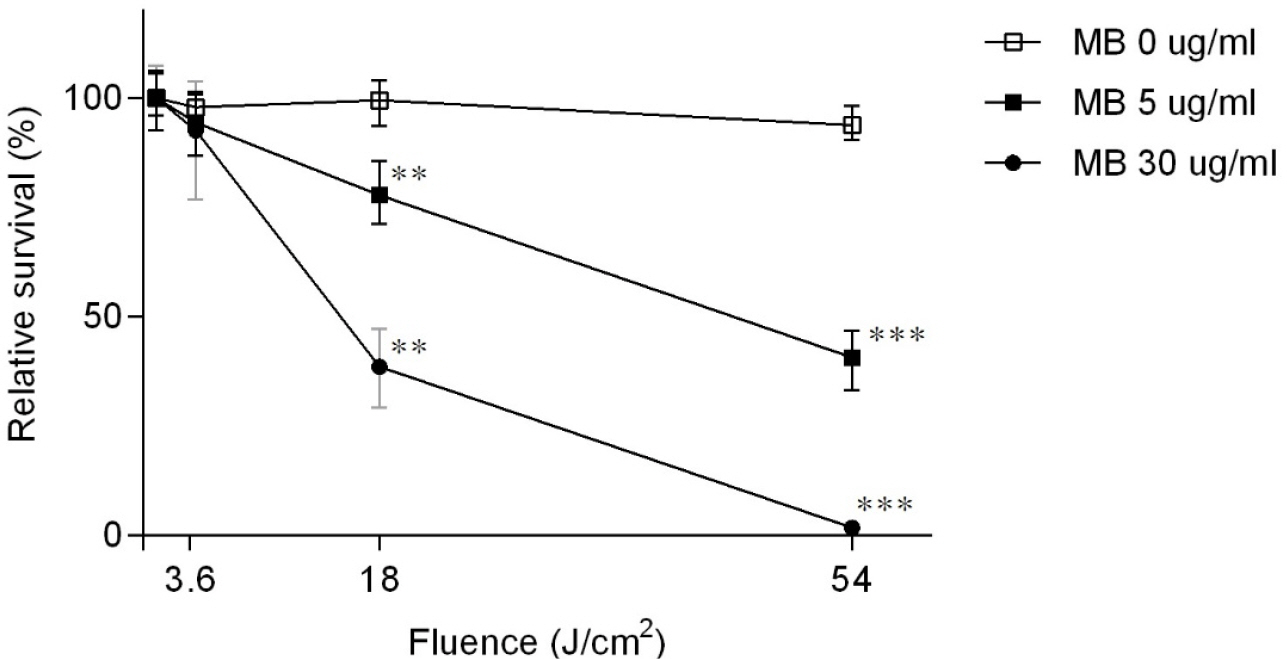
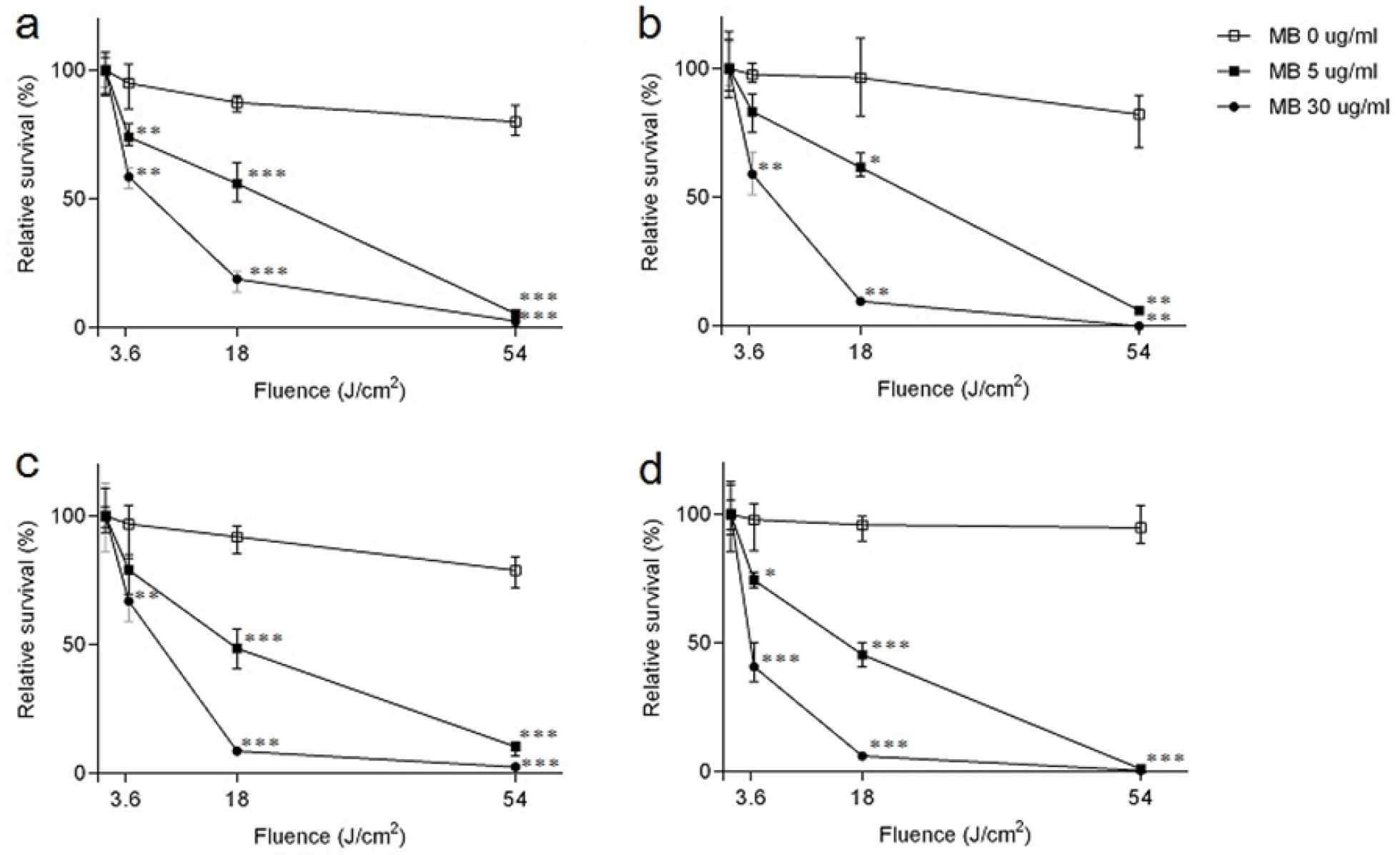
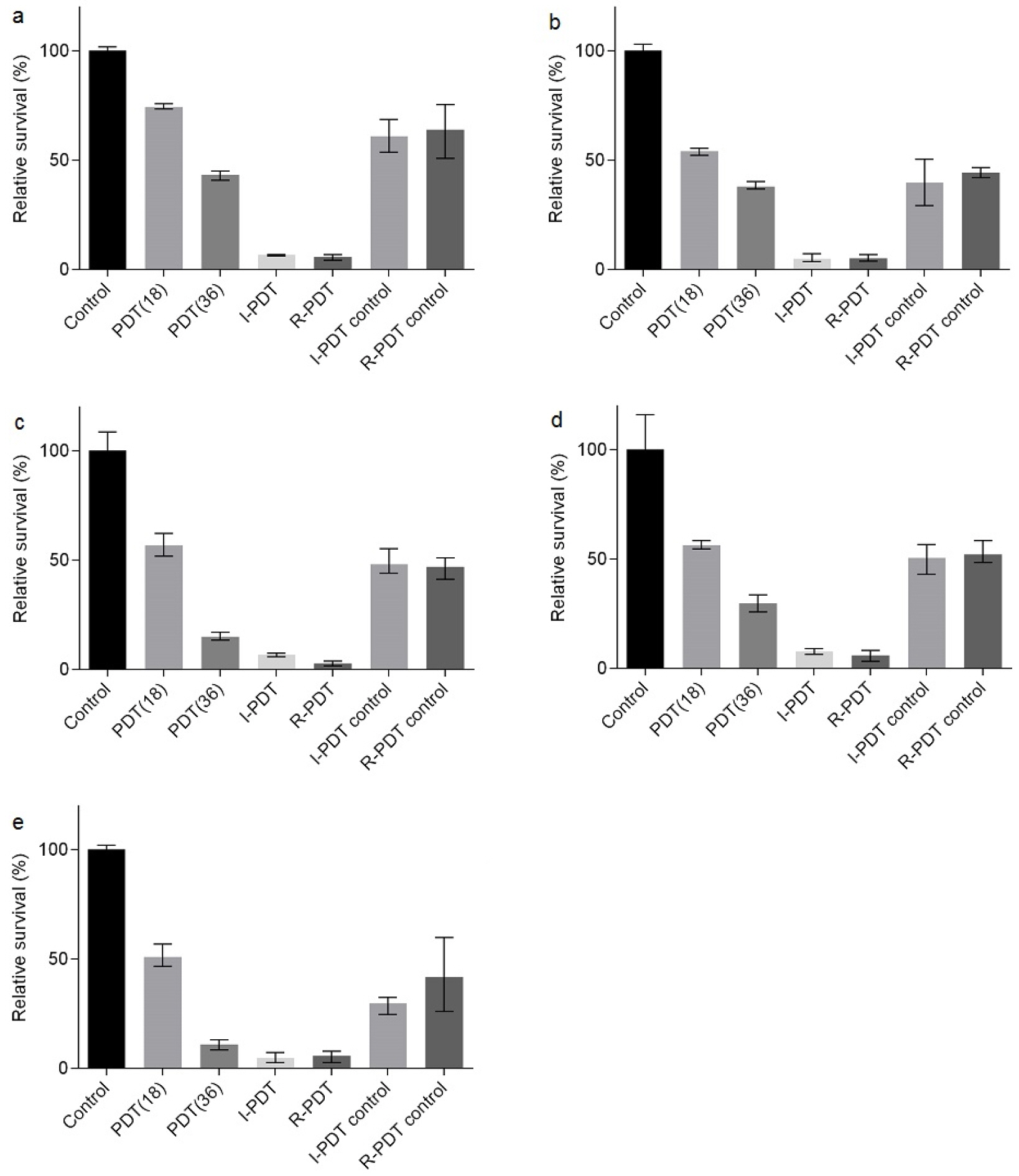
- Tuberculosis (TB) is an old disease caused by Mycobacterium tuberculosis. Although it has been known for humans for thousands of years, the treatment of this disease still requires a lengthy therapy with multiple antibiotics. Also, the emergence of multidrug-resistant strains made it more difficult to treat TB, calling for a novel treatment approach. In Photodynamic therapy (PDT), a photosensitizer, such as methylene blue (MB), is irradiated by a laser, generating reactive oxygen species and killing microorganisms. Here, using M. smegmatis as a model mycobacterium, we examined the utility of PDT in TB treatment. The photosensitizer MB alone showed weak antimicrobial activity; however, when irradiated by a laser, it efficiently killed M. smegmatis (> 97% killing with 30 mg/ml MB and 54 J/cm2 irradiation). Surprisingly, PDT showed more efficient killing activity toward drug-resistant strains of M. smegmatis than the drug-sensitive wild-type strain. In PDT, when the irradiation step alone (Intermittent PDT) or the entire PDT process was repeated (Repeated PDT), the bactericidal activity was significantly enhanced. Since PDT can be applied locally in a short period of time and kills mycobacterium irrespective of its antibiotic resistance status, we conclude that PDT can be a viable option for TB treatment.
Keyword
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2020. Licence: CC BY-NC-SA 3.0 IGO.2. LoBue P, Sizemore C, Castro K. Plan to combat extensively drug-resistant tuberculosis recommendations of the federal tuberculosis task force. MMWR Recomm Rep 2009;58:1-43.3. Korea centers for Disease Control and Prevention. Annual report on the notified tuberculosis in Korea. 2020.4. Bliss JM, Bigelow CE, Foster TH, Haidaris CG. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother 2004;48:2000-6.DOI: 10.1128/AAC.48.6.2000-2006.2004. PMID: 15155191. PMCID: PMC415596.5. Dougherty TJ. Photodynamic therapy. Photochem Photobiol 1993;58:895-900.DOI: 10.1111/j.1751-1097.1993.tb04990.x. PMID: 8310013.6. Weishaupt KR, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res 1976;36:2326-9.7. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic Therapy. J Natl Cancer Inst 1998;90:889-905.DOI: 10.1093/jnci/90.12.889. PMID: 9637138. PMCID: PMC4592754.8. Maunoury V, Mordon S, Bulois P, Mirabel X, Hecquet B, Mariette C. Photodynamic therapy for early oesophageal cancer. Dig Liver Dis 2005;37:491-5.DOI: 10.1016/j.dld.2005.02.007. PMID: 15975535.9. Cuenca RE, Allison RR, Sibata C, Downie GH. Breast cancer with chest wall progression: treatment with photodynamic therapy. Ann Surg Oncol 2004;11:322-7.DOI: 10.1245/ASO.2004.03.025. PMID: 14993029.10. Jones BU, Helmy M, Brenner M, Serna DL, Williams J, Chen JC, et al. Photodynamic therapy for patients with advanced non-small-cell carcinoma of the lung. Clin Lung Cancer 2001;3:37-41.DOI: 10.3816/CLC.2001.n.016. PMID: 14656388.11. Wilson M, Mia N. Sensitization of Candida albicans to Killing by low power laser light. J Oral Pathol Med 1993;22:354-7.DOI: 10.1111/j.1600-0714.1993.tb01088.x. PMID: 7506775.12. Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res 2009;44:751- 9.DOI: 10.1111/j.1600-0765.2008.01187.x. PMID: 19602126. PMCID: PMC2784141.13. Garland MJ, Cassidy CM, Woolfson D, Donnelly RF. Designing photosensitizers for photodynamic therapy: strategies, challenges and promising developments. Future Med Chem 2009;1:667-91.DOI: 10.4155/fmc.09.55. PMID: 21426032.14. Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, Hamblin MR. Type Ⅰ and Type Ⅱ mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg Med 2012;44:490-9.DOI: 10.1002/lsm.22045. PMID: 22760848. PMCID: PMC3428129.15. Wiegell SR, Kongshoj B, Wulf HC. Mycobacterium marinum Infection Cured by Photodynamic Therapy. Arch Dermatol 2006;142:1241-2.DOI: 10.1001/archderm.142.9.1241. PMID: 16983024.16. O'Riordan K, Sharlin DS, Gross J, Chang S, Errabelli D, Akilov OE, et al. Photoinactivation of Mycobacteria in vitro and in a new murine model of localized Mycobacterium bovis BCG-induced granulomatous infection. Antimicrob Agents Chemother 2006;50:1828-34.DOI: 10.1128/AAC.50.5.1828-1834.2006. PMID: 16641456. PMCID: PMC1472192.17. Sung N, Back S, Jung J, Kim KH, Kim JK, Lee JH, et al. Inactivation of multidrug resistant (MDR)- and extensively drug resistant (XDR)-Mycobacterium tuberculosis by photodynamic therapy. Photodiagnosis Photodyn Ther 2013;10:694-702.DOI: 10.1016/j.pdpdt.2013.09.001. PMID: 24284129.18. de Oliveira BP, Aguiar CM, Câmara AC. Photodynamic therapy in combating the causative microoraganisms from endodentic infections. Eur J Dent 2014;8:424-30.DOI: 10.4103/1305-7456.137662. PMID: 25202228. PMCID: PMC4144146.19. Klepac-Ceraj V, Patel N, Song X, Holewa C, Patel C, Kent R, et al. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers surg Med 2011;43:600-6.DOI: 10.1002/lsm.21069. PMID: 22057487. PMCID: PMC3211097.20. Siddiqui SH, Awan KH, Javed F. Bacterial efficacy of photodynamic therapy against Enterococcus faecalis in infected root canals: a systematic literature review. Photodiagnosis Photodyn Ther 2013;10:632-43.DOI: 10.1016/j.pdpdt.2013.07.006. PMID: 24192536.21. Shim I, Choi M, Min Y, Seok KH, Kim JK, Jeong JY, et al. Effect of methylene blue-mediated photodynamic therapy on wild-type and ciprofloxacin-resistant Mycobacterium smegmatis. J Bacteriol Virol 2016;46:27-35.DOI: 10.4167/jbv.2016.46.1.27.22. Titgemeyer F, Amon J, Parche S, Mahfoud M, Bail J, Schlicht M, et al. A genomic view of sugar transport in Mycobacterium smegmatis and Mycobacterium tuberculosis. J Bacteriol 2007;189:5903-15.DOI: 10.1128/JB.00257-07. PMID: 17557815. PMCID: PMC1952047.23. Liu H, Yang M, He ZG. Novel TetR family transcriptional factor regulates expression of multiple transport-related genes and affects rifampicin resistance in Mycobacterium smegmatis. Sci Rep 2016;6:27489.DOI: 10.1038/srep27489. PMID: 27271013. PMCID: PMC4895335.24. Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother 1995;39:1554-8.DOI: 10.1128/AAC.39.7.1554. PMID: 7492103. PMCID: PMC162780.25. Takiff HE, Salazar L, Guerrero C, Philipp W, Huang WM, Kreiswirth B, et al. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob Agents Chemother 1994;38:773-80.DOI: 10.1128/AAC.38.4.773. PMID: 8031045. PMCID: PMC284541.26. Anek-Vorapong R, Sinthuwattanawibool C, Podewils LJ, McCarthy K, Ngamlert K, Promsarin B, et al. Validation of the Geno Type MTBDR plus assay for detection of MDR-TB in a public health laboratory in Thailand. BMC Infect Dis 2010;10:123.DOI: 10.1186/1471-2334-10-123. PMID: 20487550. PMCID: PMC2886057.27. Hillemann D, Rüsch-Gerdes S, Richter E. Evaluation of the Geno Type MTBDR plus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 2007;45:2635-40.DOI: 10.1128/JCM.00521-07. PMID: 17537937. PMCID: PMC1951233.28. Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis 1998;79:3-29.DOI: 10.1054/tuld.1998.0002. PMID: 10645439.29. Musser JM. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev 1995;8:496-514.DOI: 10.1128/CMR.8.4.496. PMID: 8665467. PMCID: PMC172873.30. Wei CJ, Lei B, Musser JM, Tu SC. Isoniazid activation defects in recombinant Mycobacterium tuberculosis catalase- peroxidase (KatG) mutants evident in InhA inhibitor production. Antimicrob Agents Chemother 2003;47:670-5.DOI: 10.1128/AAC.47.2.670-675.2003. PMID: 12543676. PMCID: PMC151726.31. Rouse DA, DeVito JA, Li Z, Byer H, Morris SL. Site-directed mutagenesis of the katG gene of Mycobacterium tuberculosis: effects on catalase-peroxidase activities and isoniazid resistance. Mol Microbiol 1996;22:583-92.DOI: 10.1046/j.1365-2958.1996.00133.x. PMID: 8939440.32. Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol 2013;5:a012559.DOI: 10.1101/cshperspect.a012559. PMID: 23378590. PMCID: PMC3552502.33. Vecchio D, Gupta A, Huang L, Landi G, Avci P, Rodas A, et al. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob Agents Chemother 2015;59:5203-12.DOI: 10.1128/AAC.00019-15. PMID: 26077247. PMCID: PMC4538466.34. Shih MH, Huang FC. Effects of photodynamic therapy on rapidly growing nontuberculous mycobacteria keratitis. Invest Ophthalmol Vis Sci 2011;52:223-9.DOI: 10.1167/iovs.10-5593. PMID: 20811055.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Methylene Blue-mediated Photodynamic Therapy on Wild-type and Ciprofloxacin-resistant Mycobacterium smegmatis
- Microgel-Encapsulated Methylene Blue for the Treatment of Breast Cancer Cells by Photodynamic Therapy
- Effects of methylene blue or evan's blue on mouse embryo development, human sperm motility, serum E2 level and components of human intra tubal fluid
- The Effect after Intra-dermal Methylene Blue, Hydrocortisone, Lidocaine Injection Therapy for Intractable, Idiopathic Pruritus Ani
- Methylene Blue