Cancer Res Treat.
2021 Jan;53(1):184-198. 10.4143/crt.2020.192.
Long Non-coding RNA CASC15 Promotes Intrahepatic Cholangiocarcinoma Possibly through Inducing PRDX2/PI3K/AKT Axis
- Affiliations
-
- 1Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Key Laboratory of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Tumor of Zhejiang Province, Hangzhou, China
- 3Research Center of Diagnosis and Treatment Technology for Hepatocellular Carcinoma of Zhejiang Province, Hangzhou, China
- 4Clinical Medicine Innovation Center of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Disease of Zhejiang University, Hangzhou, China
- 5Clinical Research Center of Hepatobiliary and Pancreatic Diseases of Zhejiang Province, Hangzhou, China
- KMID: 2510660
- DOI: http://doi.org/10.4143/crt.2020.192
Abstract
- Purpose
Intrahepatic cholangiocarcinoma (ICC) is one of the most common liver primary tumors but its treatments are limited. Bioinformatics showed that the expression level of long non-coding RNA cancer-associated susceptibility 15 gene (CASC15) is correlated with ICC progression, but its functional mechanism remains unclear.
Materials and Methods
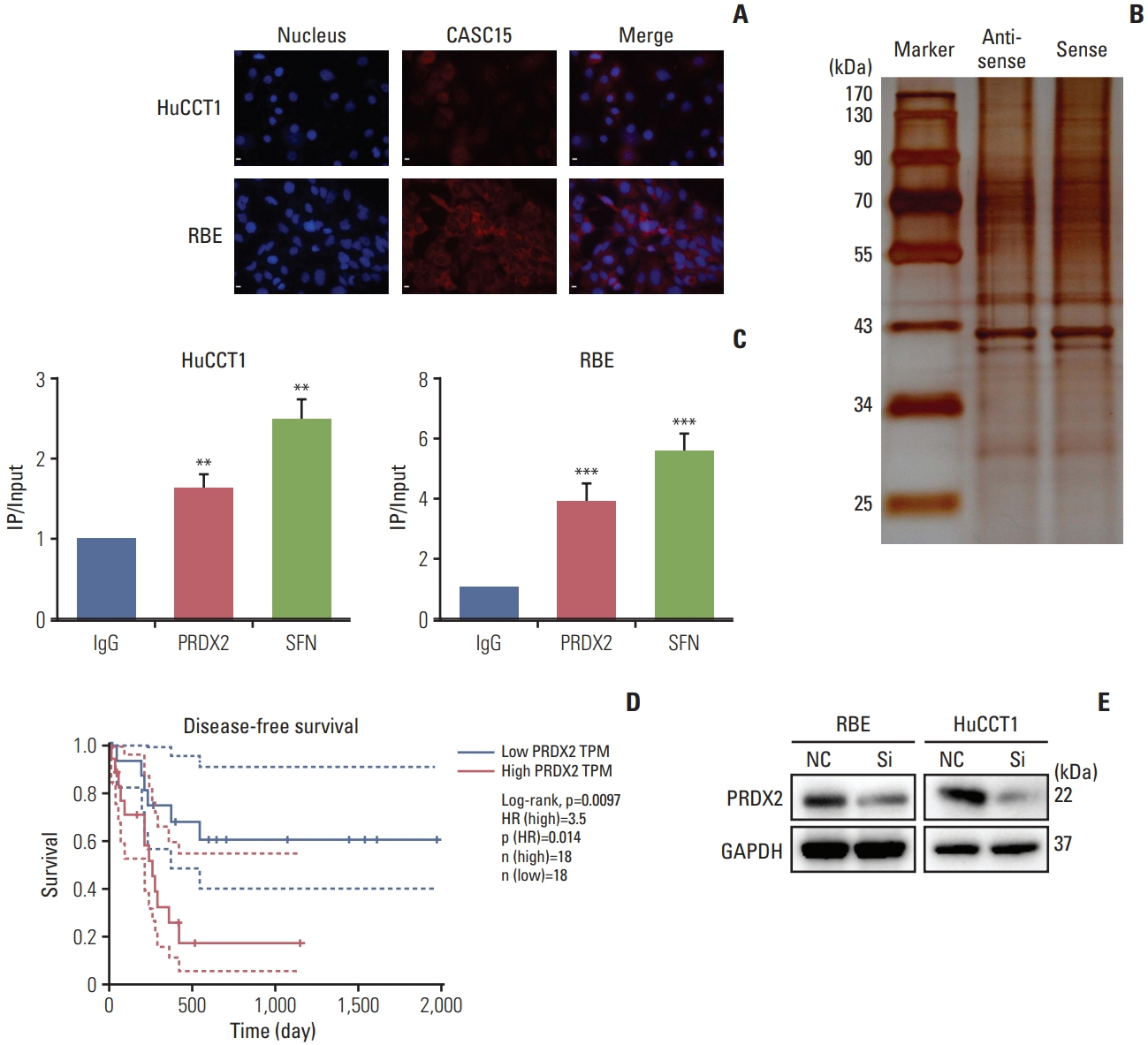
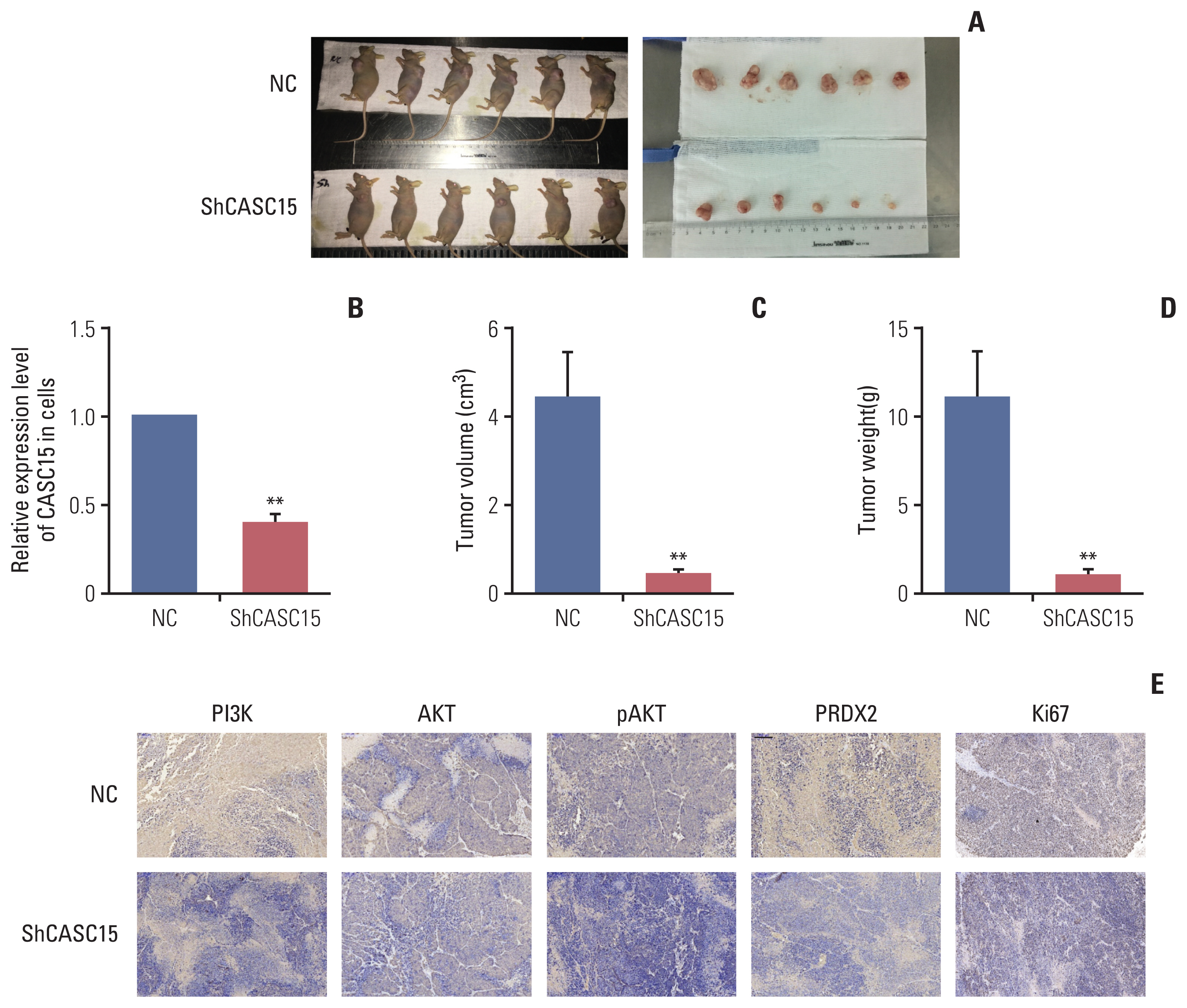
Tissues from ICC patients, tumor and adjacent tissue, were used for detection of the expression of CASC15. Clinical data were also collected for clinicopathologic and survival analysis. Short interfering RNA and lentiviral short hairpin RNA were used to knock down CASC15 and PRDX2 expression in ICC cell lines, for the analysis of changes of cell function and xenografts. RNA-pulldown and RNA immunoprecipitation assays were used to detect RNA-binding protein, PRDX2. Male nude mice were used for ICC xenografts, and livers were collected after 4 weeks for immunohistochemistry.
Results
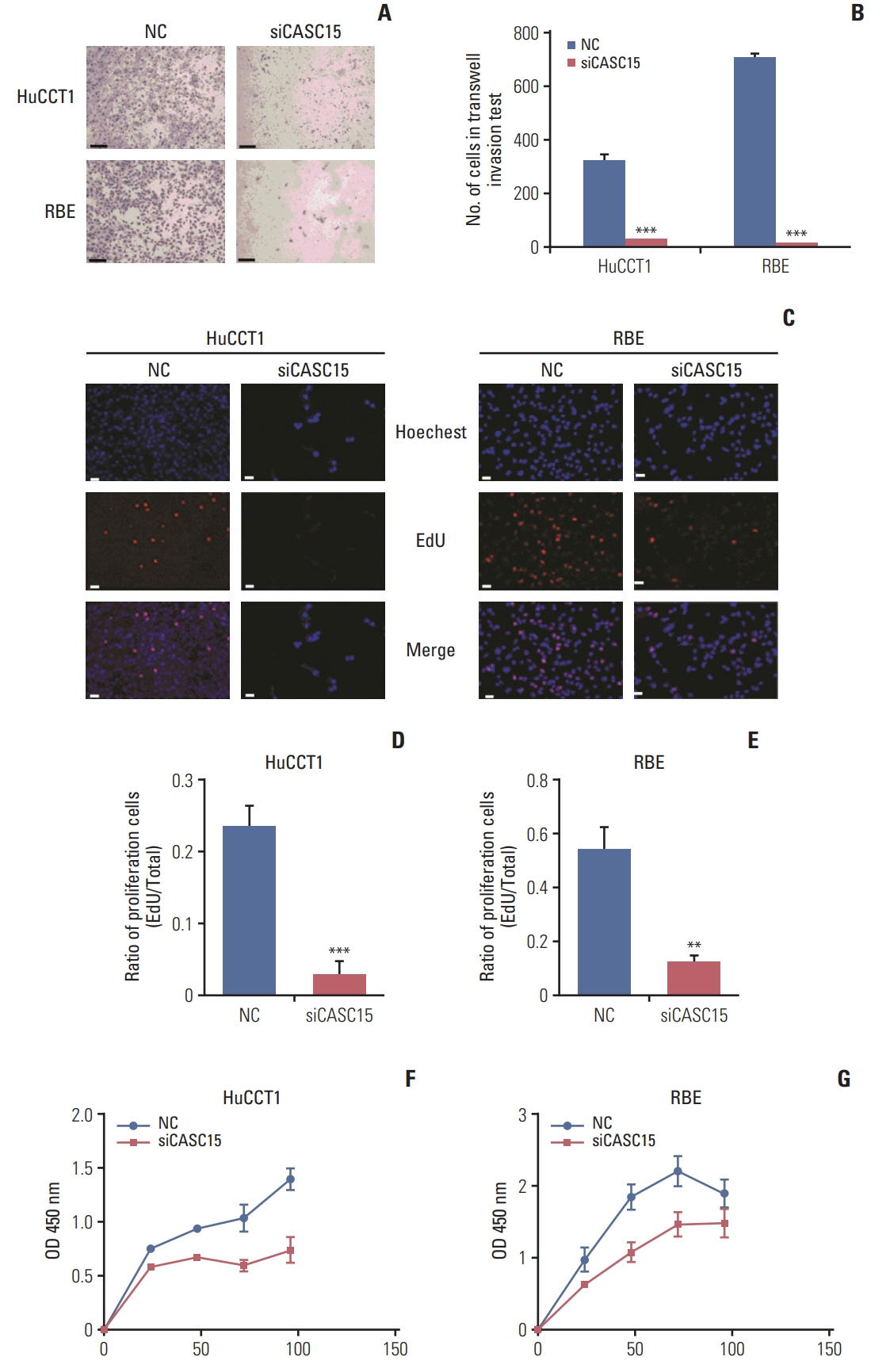
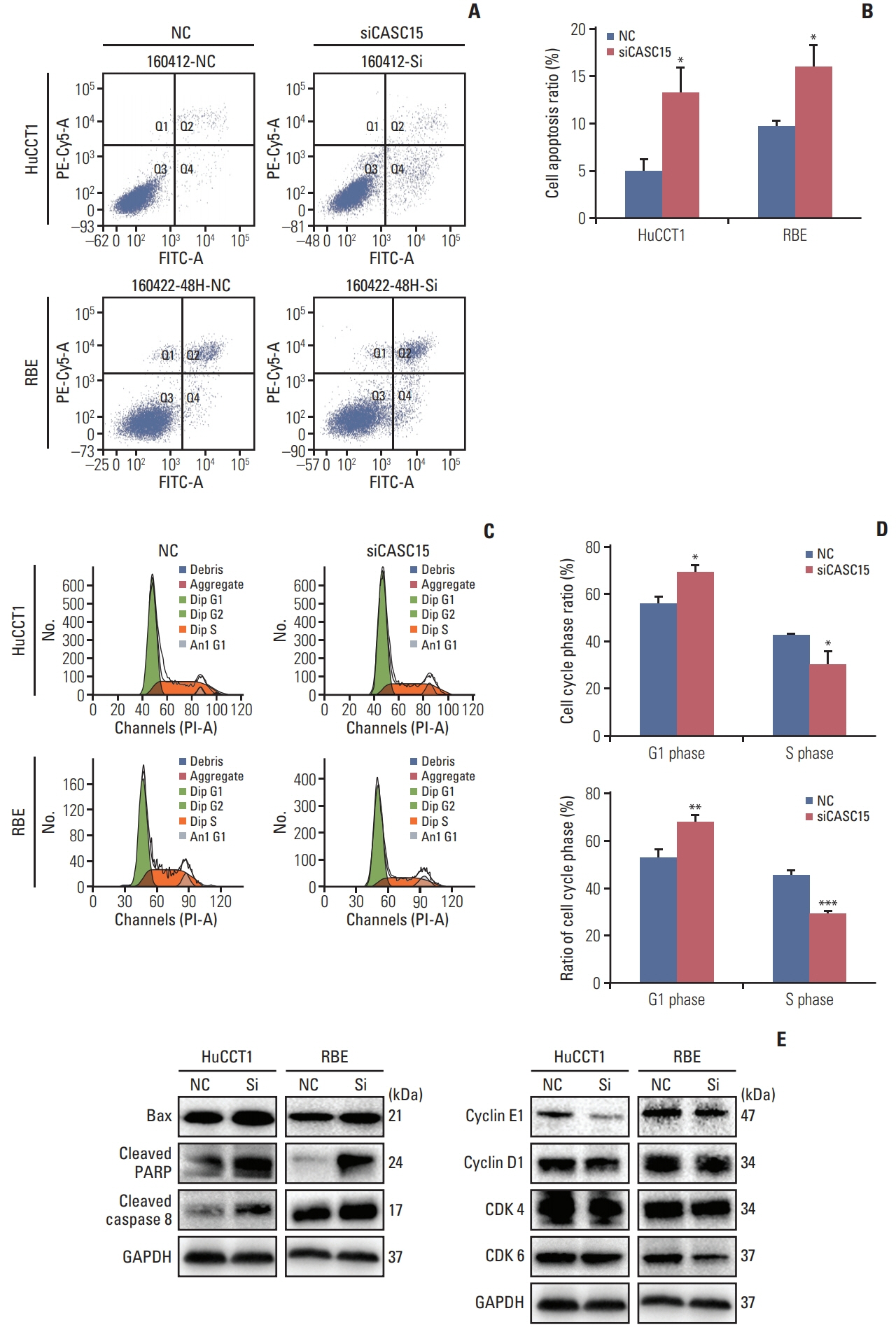
CASC15 is highly expressed in ICC tissues and is related to higher TNM stage. Knockdown of CASC15 in ICC cells reduced cell proliferation, migration, invasiveness and increased apoptosis, and G1/S block. PRDX2 bound to CASC15. Knockdown of CASC15 decreased PRDX2 expression which was rescued by the inhibition of proteasome formation. Downregulation of PRDX2 resulted in G1/S block, reduced ICC cell invasion. Downregulation of CASC15 inhibited phosphoinositide 3-kinase (PI3K)/AKT/c-Myc pathway through downregulating of PRDX2 and overexpressed PRDX2 rescued the block. CASC15 knockout in ICC xenografts suppressed tumor development in vivo, decreased the expression of PRDX2 and Ki67 and inhibited PI3K/AKT pathway.
Conclusion
CASC15 promotes ICC possibly by targeting PRDX2 via the PI3K/AKT pathway, indicating poor prognosis and high degree of malignancy of ICC.
Figure
Reference
-
References
1. Mao K, Jiang W, Liu J, Wang J. Incidence of subsequent cholangiocarcinomas after another malignancy: trends in a population-based study. Medicine (Baltimore). 2015; 94:e596.2. Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008; 48:308–21.
Article3. Buettner S, van Vugt JL, IJzermans JM, Groot Koerkamp B. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther. 2017; 10:1131–42.
Article4. Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004; 9:43–57.
Article5. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009; 461:747–53.
Article6. Bertone P, Stolc V, Royce TE, Rozowsky JS, Urban AE, Zhu X, et al. Global identification of human transcribed sequences with genome tiling arrays. Science. 2004; 306:2242–6.
Article7. Zhang A, Zhao JC, Kim J, Fong KW, Yang YA, Chakravarti D, et al. LncRNA HOTAIR enhances the androgen-receptor-mediated transcriptional program and drives castration-resistant prostate cancer. Cell Rep. 2015; 13:209–21.
Article8. Gao S, Wang P, Hua Y, Xi H, Meng Z, Liu T, et al. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget. 2016; 7:1608–18.
Article9. He T, Zhang L, Kong Y, Huang Y, Zhang Y, Zhou D, et al. Long non-coding RNA CASC15 is upregulated in hepatocellular carcinoma and facilitates hepatocarcinogenesis. Int J Oncol. 2017; 51:1722–30.
Article10. Merdrignac A, Angenard G, Allain C, Petitjean K, Bergeat D, Bellaud P, et al. A novel transforming growth factor beta-induced long noncoding RNA promotes an inflammatory microenvironment in human intrahepatic cholangiocarcinoma. Hepatol Commun. 2018; 2:254–69.
Article11. Jiang F, Ling X. The Advancement of long non-coding RNAs in cholangiocarcinoma development. J Cancer. 2019; 10:2407–14.
Article12. Bertoldi M. Human peroxiredoxins 1 and 2 and their interacting protein partners: through structure toward functions of biological complexes. Protein Pept Lett. 2016; 23:69–77.
Article13. Castaldo SA, Ajime T, Serrao G, Anastacio F, Rosa JT, Giacomantonio CA, et al. Annexin A2 regulates AKT upon H2O2-dependent signaling activation in cancer cells. Cancers (Basel). 2019; 11:492.
Article14. Jin Y, Yang Q, Liang L, Ding L, Liang Y, Zhang D, et al. Compound kushen injection suppresses human acute myeloid leukaemia by regulating the Prdxs/ROS/Trx1 signalling pathway. J Exp Clin Cancer Res. 2018; 37:277.
Article15. Zhang Y, Sun C, Xiao G, Shan H, Tang L, Yi Y, et al. S-nitrosylation of the peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death Dis. 2019; 10:329.
Article16. Luthra S, Chandran U, Diergaarde B, Becich M, Lee AV, Neumann CA. Expression of reactive species related genes is associated with patient survival in luminal B breast cancer. Free Radic Biol Med. 2018; 120:170–80.
Article17. Gu C, Luo J, Lu X, Tang Y, Ma Y, Yun Y, et al. REV7 confers radioresistance of esophagus squamous cell carcinoma by recruiting PRDX2. Cancer Sci. 2019; 110:962–72.
Article18. Xu J, Zhang S, Wang R, Wu X, Zeng L, Fu Z. Knockdown of PRDX2 sensitizes colon cancer cells to 5-FU by suppressing the PI3K/AKT signaling pathway. Biosci Rep. 2017; 37:BSR20160447.
Article19. Xu D, Yang F, Yuan JH, Zhang L, Bi HS, Zhou CC, et al. Long noncoding RNAs associated with liver regeneration 1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/beta-catenin signaling. Hepatology. 2013; 58:739–51.20. Togami K, Yamaguchi K, Chono S, Tada H. Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-beta1 in A549, NCI-H441, and Calu-3 cells: development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis. J Pharmacol Toxicol Methods. 2017; 86:19–27.21. Richards EJ, Zhang G, Li ZP, Permuth-Wey J, Challa S, Li Y, et al. Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) beta: lncRNA-hit-mediated TGFbeta-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem. 2015; 290:6857–67.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-21 promotes epithelial-mesenchymal transition and migration of human bronchial epithelial cells by targeting poly (ADP-ribose) polymerase-1 and activating PI3K/AKT signaling
- MLL4 Regulates the Progression of Non–Small-Cell Lung Cancer by Regulating the PI3K/AKT/SOX2 Axis
- CDK1 promotes the phosphorylation of KIFC1 to regulate the tumorgenicity of endometrial carcinoma
- Arctigenin Increases Hemeoxygenase-1 Gene Expression by Modulating PI3K/AKT Signaling Pathway in Rat Primary Astrocytes
- Long Non-Coding RNA LINC00525 Promotes the Stemness and Chemoresistance of Colorectal Cancer by Targeting miR-507/ELK3 Axis