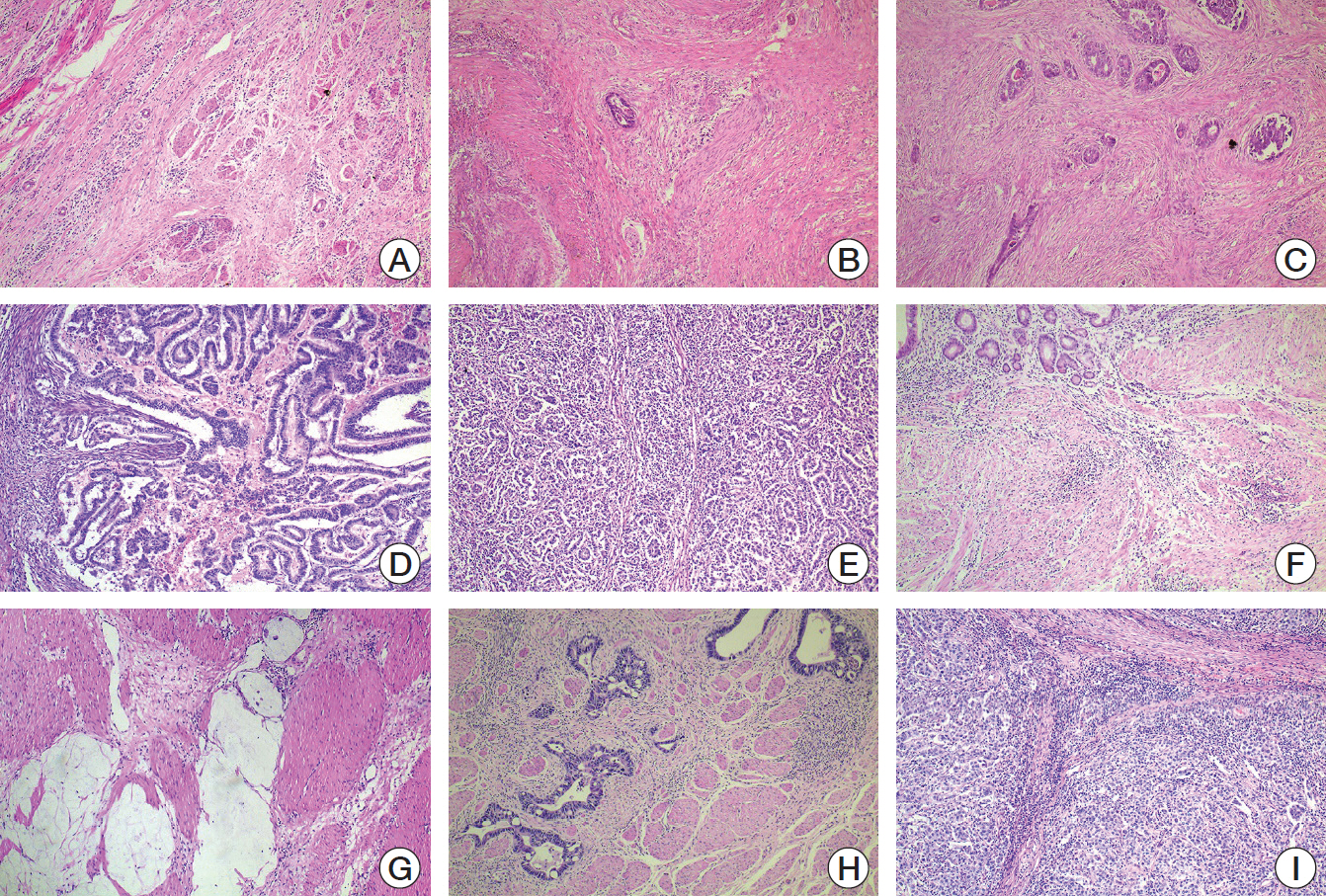
Cancer Res Treat.
2021 Jan;53(1):112-122. 10.4143/crt.2020.516.
Evaluation and Comparison of Predictive Value of Tumor Regression Grades according to Mandard and Becker in Locally Advanced Gastric Adenocarcinoma
- Affiliations
-
- 1Department of Gastric Surgery, Liaoning Cancer Hospital and Institute, Cancer Hospital of China Medical University, Shenyang, China
- 2Department of Pathology, Liaoning Cancer Hospital and Institute, Cancer Hospital of China Medical University, Shenyang, China
- KMID: 2510653
- DOI: http://doi.org/10.4143/crt.2020.516
Abstract
- Purpose
Tumor regression grade (TRG) has been widely used in gastrointestinal carcinoma to assess pathological responses to neoadjuvant chemotherapy (NCT). There are various standards without a consensus, and it is still unclear which kind of system has better predictive value. This study aims to investigate and compare the predictive ability of the Mandard and Becker TRGs in patients with locally advanced gastric cancer.
Materials and Methods
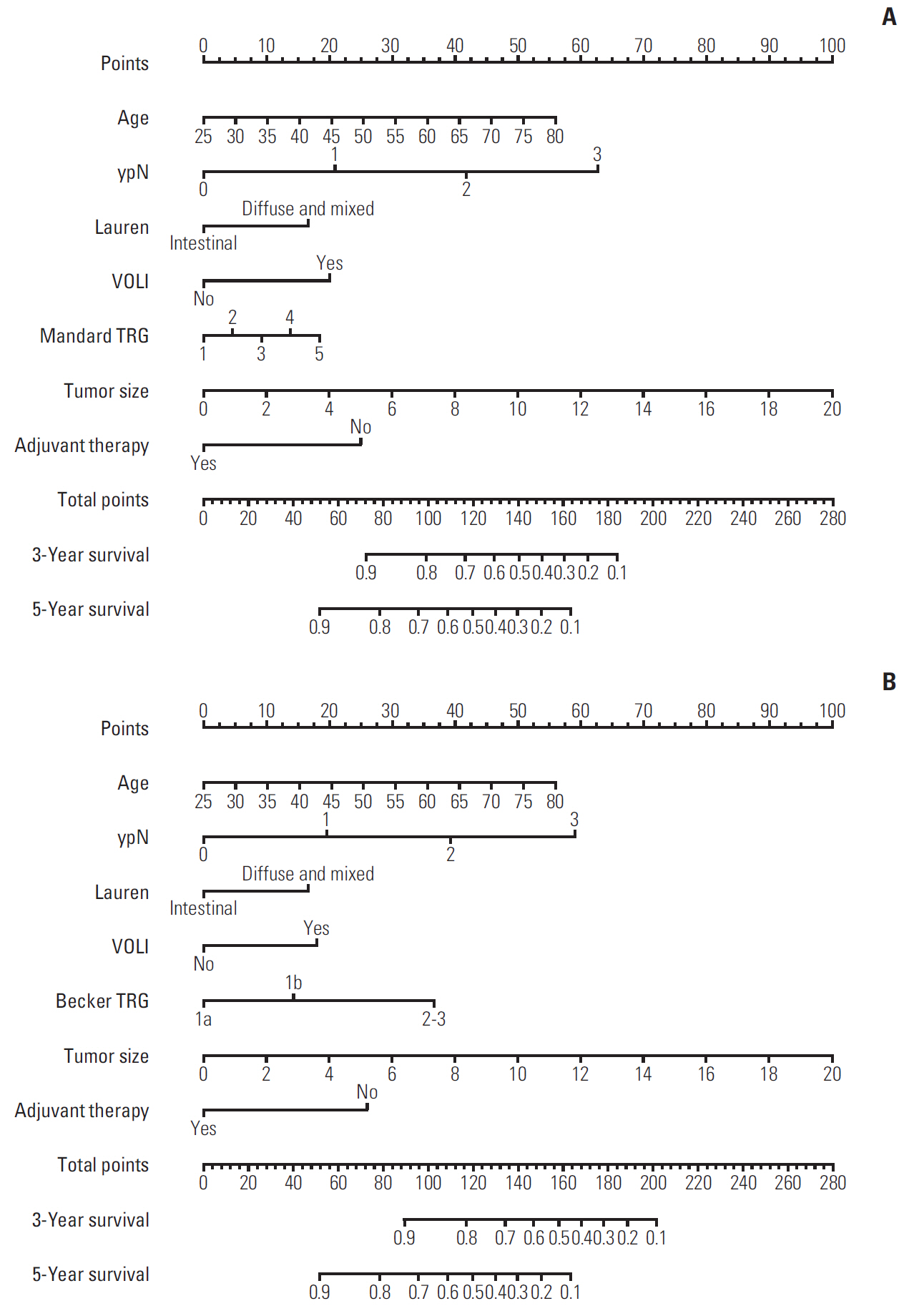
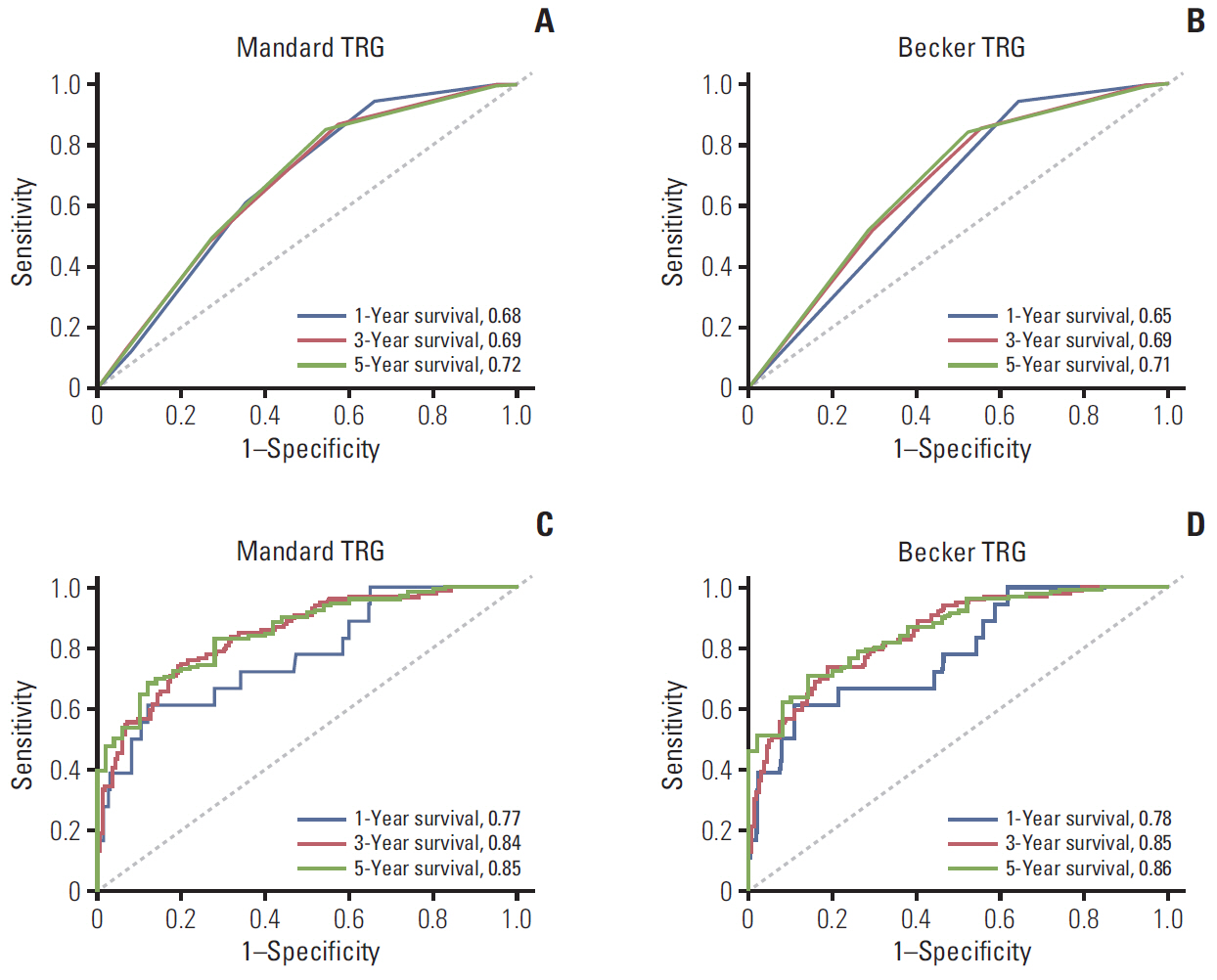
A total of 290 patients with locally advanced gastric adenocarcinoma who underwent NCT and curative surgery were studied. Survival analysis for overall survival (OS) and disease-free survival (DFS) were based on the Kaplan-Meier method and Cox proportional hazards method. Predictive values of TRGs and models were assessed by time-dependent receiver operating characteristic (ROC) curve, the area under the ROC curve (AUC), nomogram, and calibration curve.
Results
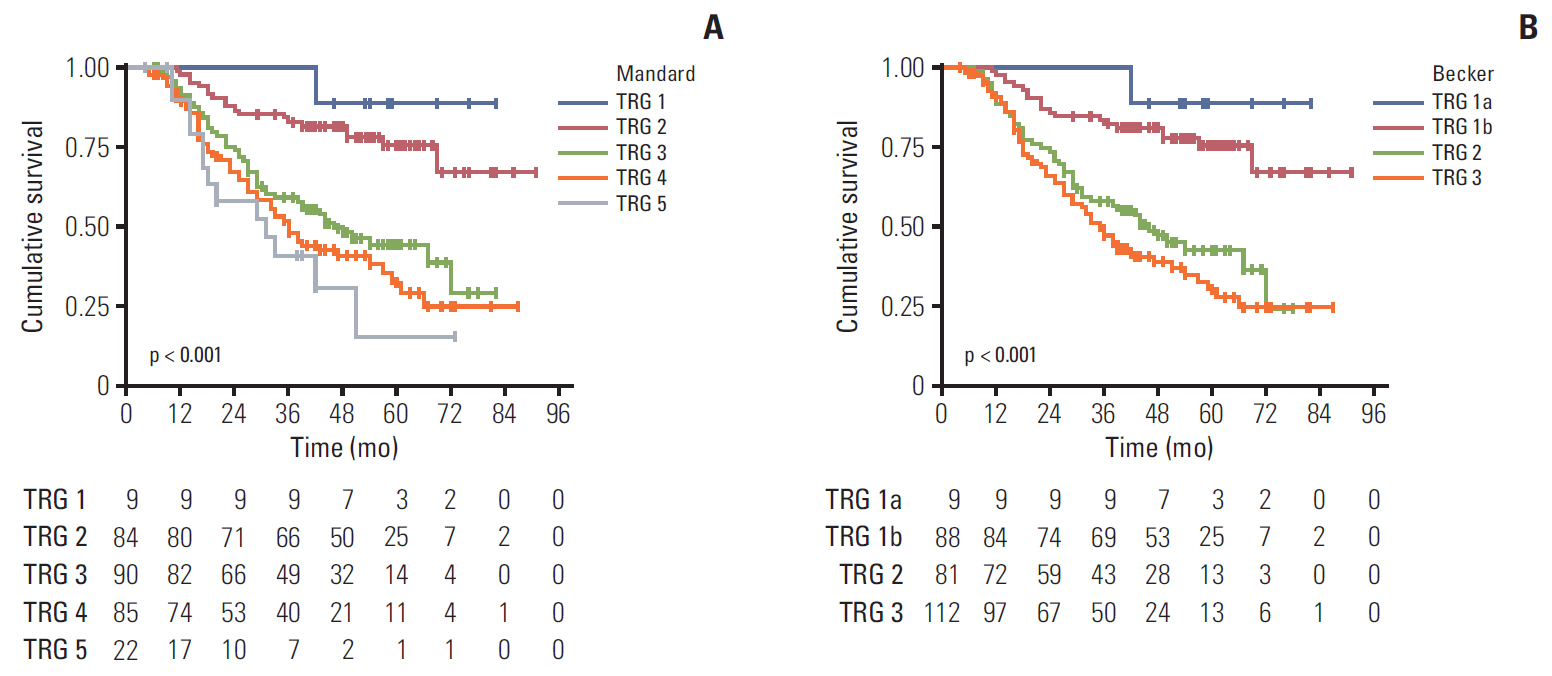
In multivariable analysis, the Mandard TRG was associated with OS (hazard ratio [HR], 1.806; p=0.026) and DFS (HR, 1.792; p=0.017). The Becker TRG was also related to OS (HR, 1.880; p=0.014) and DFS (HR, 1.919; p=0.006). The Mandard and Becker TRG AUCs for 5-year survival were 0.72 and 0.71, respectively. The whole models showed an increased predictive value, with AUCs of 0.85 and 0.86, respectively. There was no significant difference between the two TRGs and two models.
Conclusion
TRG was an independent predictor for survival, and there was no significant difference between these two systems.
Keyword
Figure
Reference
-
References
1. Martin-Romano P, Sola JJ, Diaz-Gonzalez JA, Chopitea A, Iragorri Y, Martinez-Regueira F, et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br J Cancer. 2016; 115:655–63.
Article2. Davies AR, Gossage JA, Zylstra J, Mattsson F, Lagergren J, Maisey N, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014; 32:2983–90.
Article3. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014; 32:1554–62.
Article4. Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, et al. Effect of pathologic tumor response and nodal status on survival in the medical research council adjuvant gastric infusional chemotherapy Trial. J Clin Oncol. 2016; 34:2721–7.
Article5. Song C, Chung JH, Kang SB, Kim DW, Oh HK, Lee HS, et al. Impact of tumor regression grade as a major prognostic factor in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: a proposal for a modified staging system. Cancers (Basel). 2018; 10:319.
Article6. Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011; 253:934–9.7. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer. 1994; 73:2680–6.
Article8. Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005; 47:141–6.
Article9. Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003; 98:1521–30.
Article10. Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005; 23:8688–96.11. Trakarnsanga A, Gonen M, Shia J, Nash GM, Temple LK, Guillem JG, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014; 106:dju248.
Article12. Langer R, Becker K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018; 472:175–86.
Article13. Rullier A, Laurent C, Capdepont M, Vendrely V, Bioulac-Sage P, Rullier E. Impact of tumor response on survival after radiochemotherapy in locally advanced rectal carcinoma. Am J Surg Pathol. 2010; 34:562–8.
Article14. Wang X, Zhao L, Liu H, Zhong D, Liu W, Shan G, et al. A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer. 2016; 114:1326–33.
Article15. Donohoe CL, O’Farrell NJ, Grant T, King S, Clarke L, Muldoon C, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg. 2013; 258:784–92.16. Karagkounis G, Thai L, Mace AG, Wiland H, Pai RK, Steele SR, et al. Prognostic implications of pathological response to neoadjuvant chemoradiation in pathologic stage III rectal cancer. Ann Surg. 2019; 269:1117–23.
Article17. Schmidt T, Sicic L, Blank S, Becker K, Weichert W, Bruckner T, et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer. 2014; 110:1712–20.
Article18. Noble F, Lloyd MA, Turkington R, Griffiths E, O’Donovan M, O’Neill JR, et al. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br J Surg. 2017; 104:1816–28.19. Mokadem I, Dijksterhuis WP, van Putten M, Heuthorst L, de Vos-Geelen JM, Haj Mohammad N, et al. Recurrence after preoperative chemotherapy and surgery for gastric adenocarcinoma: a multicenter study. Gastric Cancer. 2019; 22:1263–73.
Article20. Fareed KR, Ilyas M, Kaye PV, Soomro IN, Lobo DN, Parsons SL, et al. Tumour regression grade (TRG) analyses in patients with resectable gastro-oesophageal adenocarcinomas treated with platinum-based neoadjuvant chemotherapy. Histopathology. 2009; 55:399–406.
Article21. Mancini R, Pattaro G, Diodoro MG, Sperduti I, Garufi C, Stigliano V, et al. Tumor regression grade after neoadjuvant chemoradiation and surgery for low rectal cancer evaluated by multiple correspondence analysis: ten years as minimum follow-up. Clin Colorectal Cancer. 2018; 17:e13–9.
Article22. Fareed KR, Al-Attar A, Soomro IN, Kaye PV, Patel J, Lobo DN, et al. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastrooesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer. 2010; 102:1600–7.
Article23. Achilli P, De Martini P, Ceresoli M, Mari GM, Costanzi A, Maggioni D, et al. Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study. J Gastrointest Oncol. 2017; 8:1018–25.
Article24. Holscher AH, Drebber U, Schmidt H, Bollschweiler E. Prognostic classification of histopathologic response to neoadjuvant therapy in esophageal adenocarcinoma. Ann Surg. 2014; 260:779–84.25. Schneider PM, Baldus SE, Metzger R, Kocher M, Bongartz R, Bollschweiler E, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg. 2005; 242:684–92.26. Swisher SG, Hofstetter W, Wu TT, Correa AM, Ajani JA, Komaki RR, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg. 2005; 241:810–7.
Article27. Puetz K, Bollschweiler E, Semrau R, Monig SP, Holscher AH, Drebber U. Neoadjuvant chemoradiation for patients with advanced oesophageal cancer: which response grading system best impacts prognostic discrimination? Histopathology. 2019; 74:731–43.28. Karamitopoulou E, Thies S, Zlobec I, Ott K, Feith M, Slotta-Huspenina J, et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am J Surg Pathol. 2014; 38:1551–6.29. Tomasello G, Petrelli F, Ghidini M, Pezzica E, Passalacqua R, Steccanella F, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: a meta-analysis of 17 published studies. Eur J Surg Oncol. 2017; 43:1607–16.
Article30. Kong JC, Guerra GR, Warrier SK, Lynch AC, Michael M, Ngan SY, et al. Prognostic value of tumour regression grade in locally advanced rectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2018; 20:574–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of Primary Tumor FDG Uptake with Histopathologic Features of Advanced Gastric Cancer
- Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer
- Two cases of mucinous adenocarcinoma of the stomach mistaken as submucosal tumor
- Expression of Sialosyl Tn Mucin Antigen in Gastric Adenocarcinoma and Its Relationship with Prognostic Factors
- Clinical Outcome of Doublet and Triplet Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer