Int J Stem Cells.
2020 Nov;13(3):404-413. 10.15283/ijsc20052.
Upregulation of C-Reactive Protein by Placenta-Derived Mesenchymal Stem Cells Promotes Angiogenesis in A Rat Model with Cirrhotic Liver
- Affiliations
-
- 1Department of Biomedical Science, CHA University, Seongnam, Korea
- 2Non-Clinical Evaluation Center, CHA Advanced Research Institute, Seongnam, Korea
- 3Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 4Department of Internal Medicine, Catholic University Medical College, Seoul, Korea
- KMID: 2508914
- DOI: http://doi.org/10.15283/ijsc20052
Abstract
- Background and Objectives
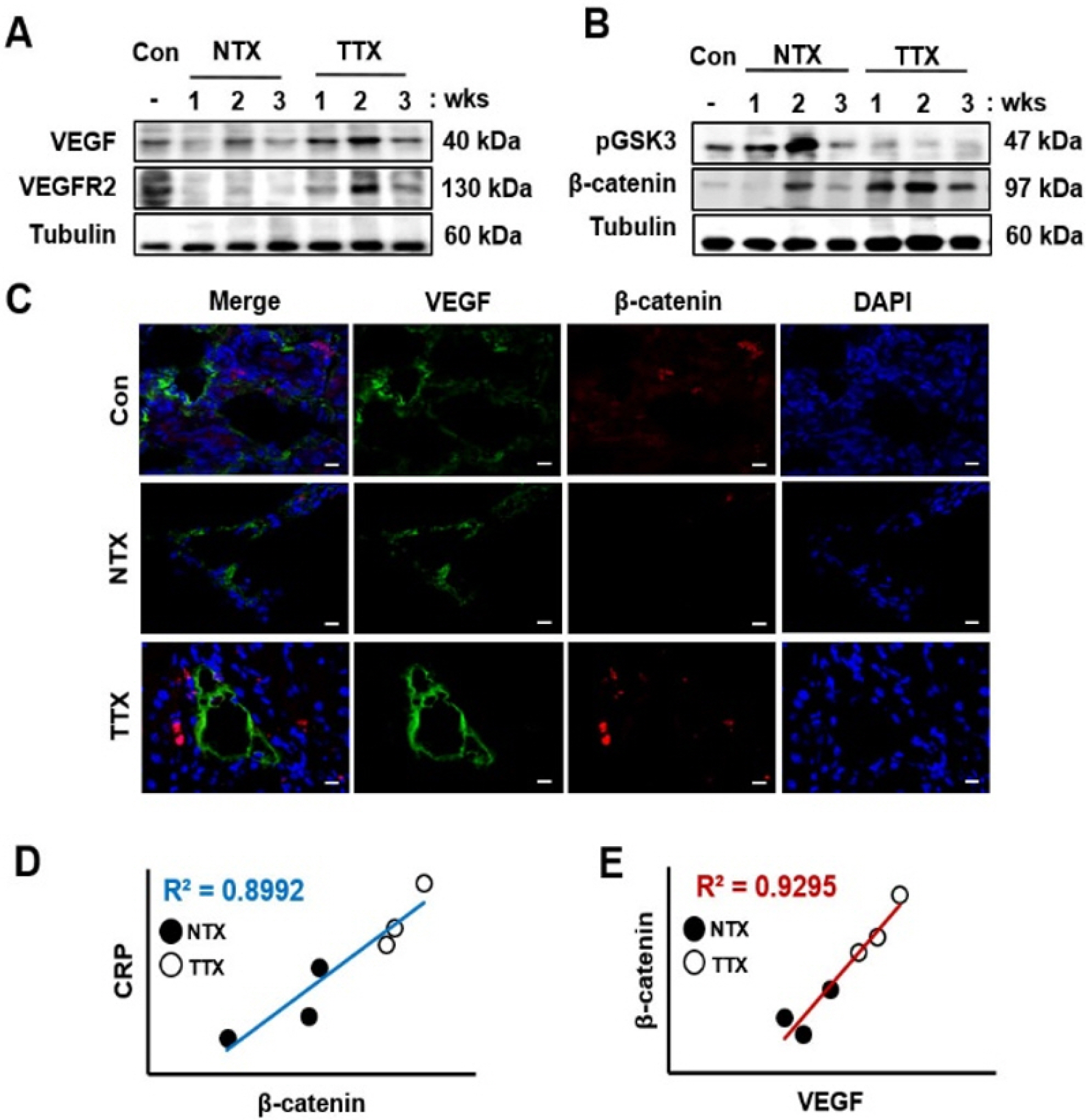
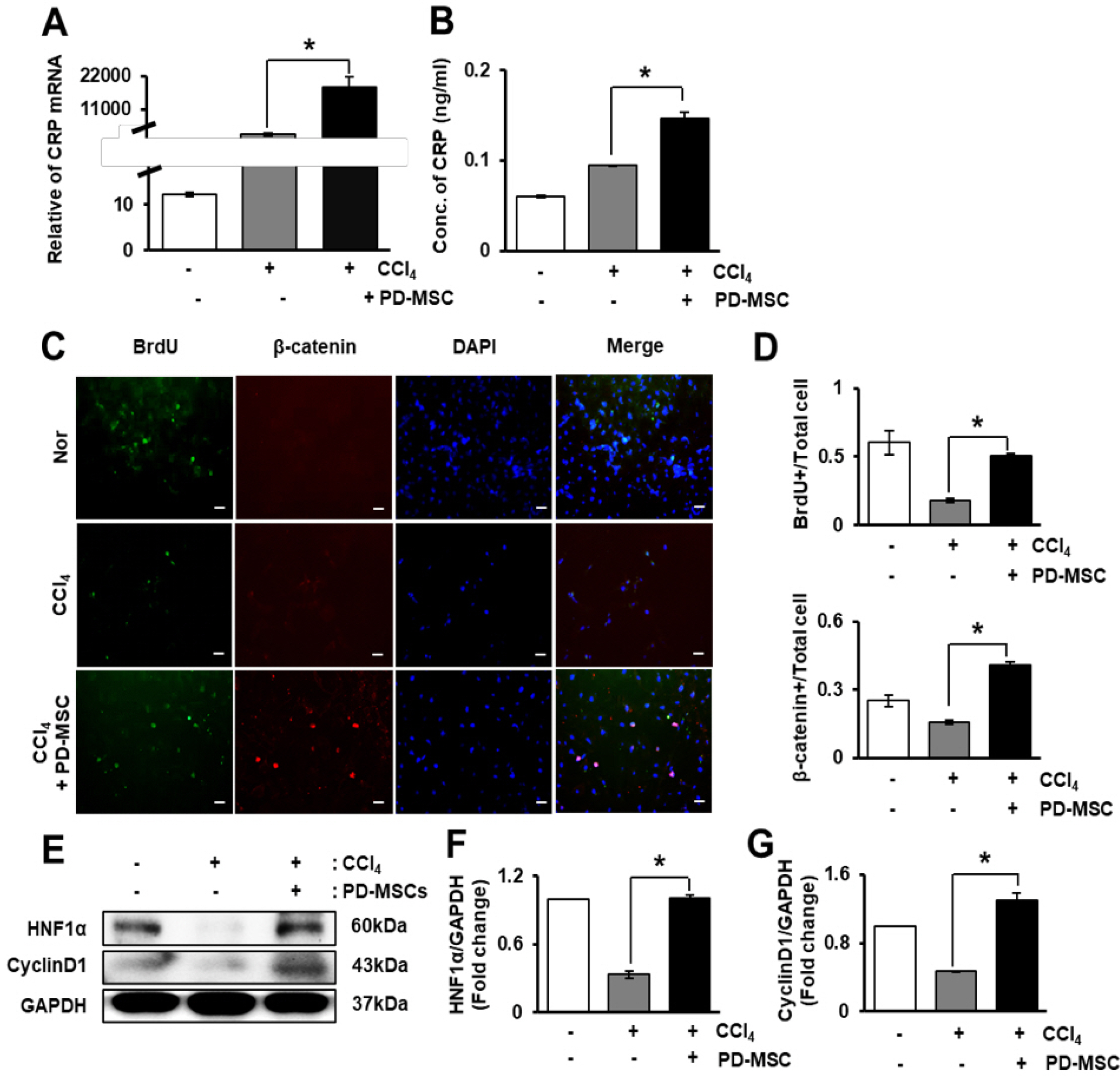
Liver cirrhosis is accompanied by abnormal vascular shunts. The Wnt pathway is essential for endothelial cell survival and proliferation. C-reactive protein (CRP), which is produced by hepatocyte, activates angiogenesis in cardiovascular diseases.
Methods and Results
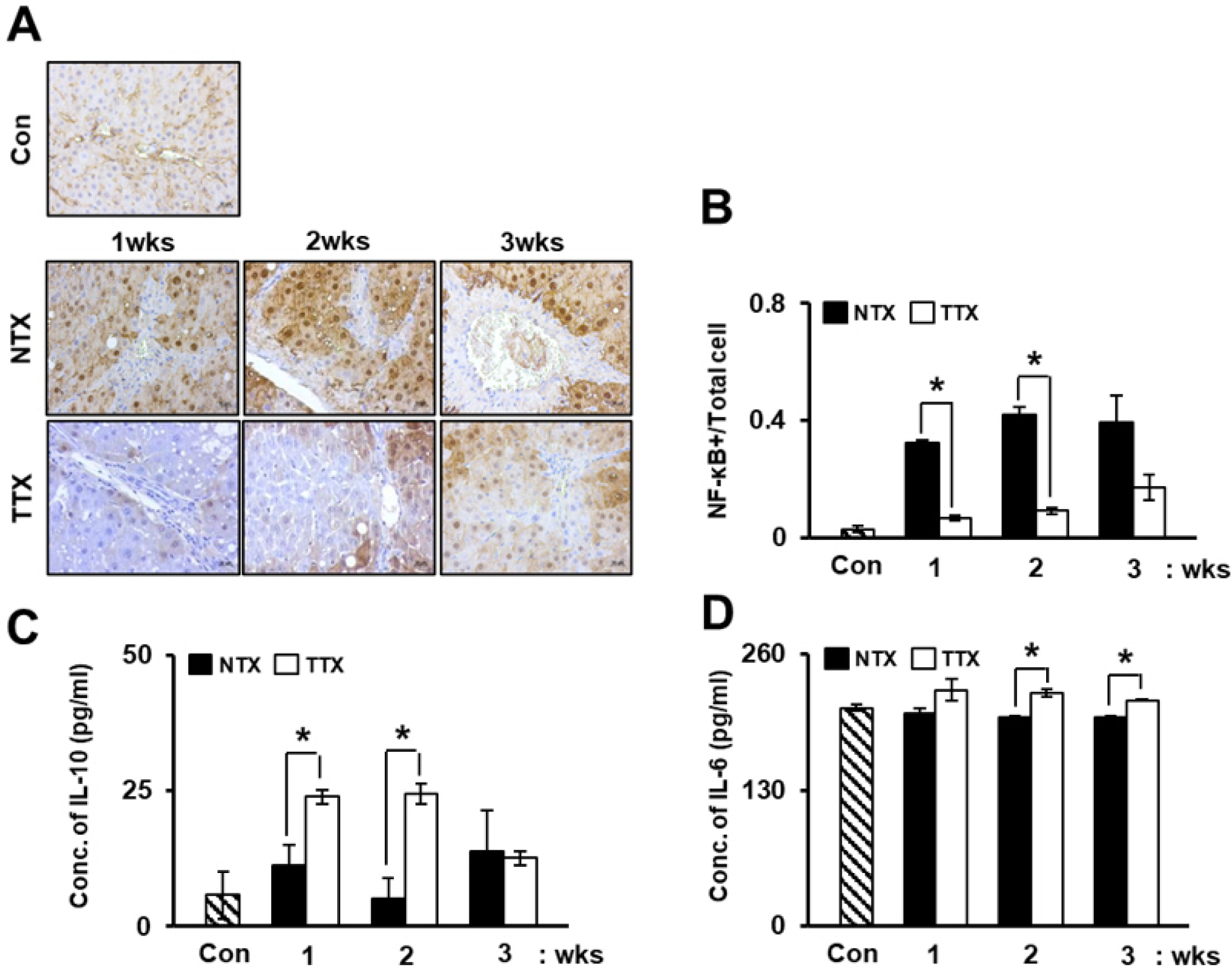
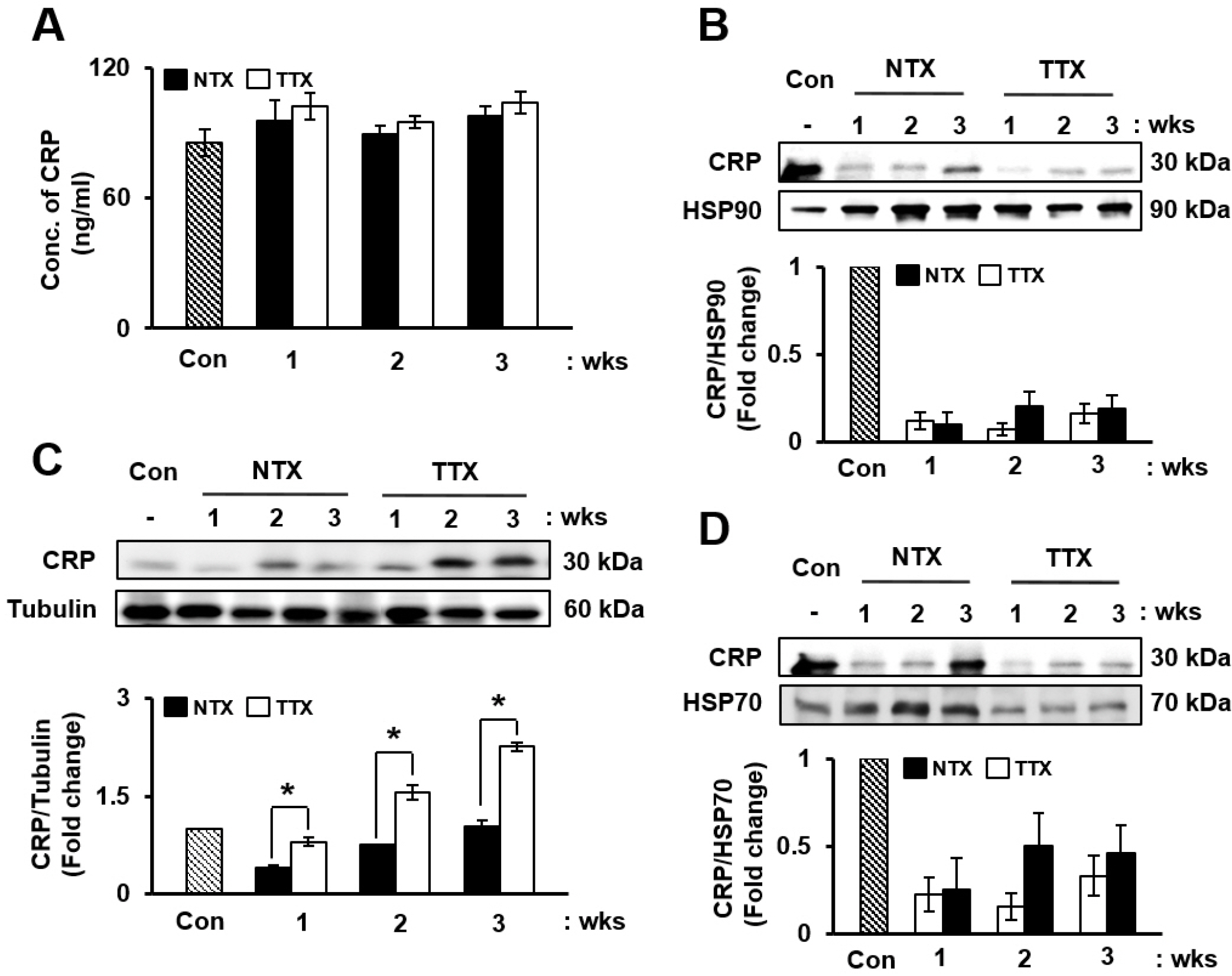
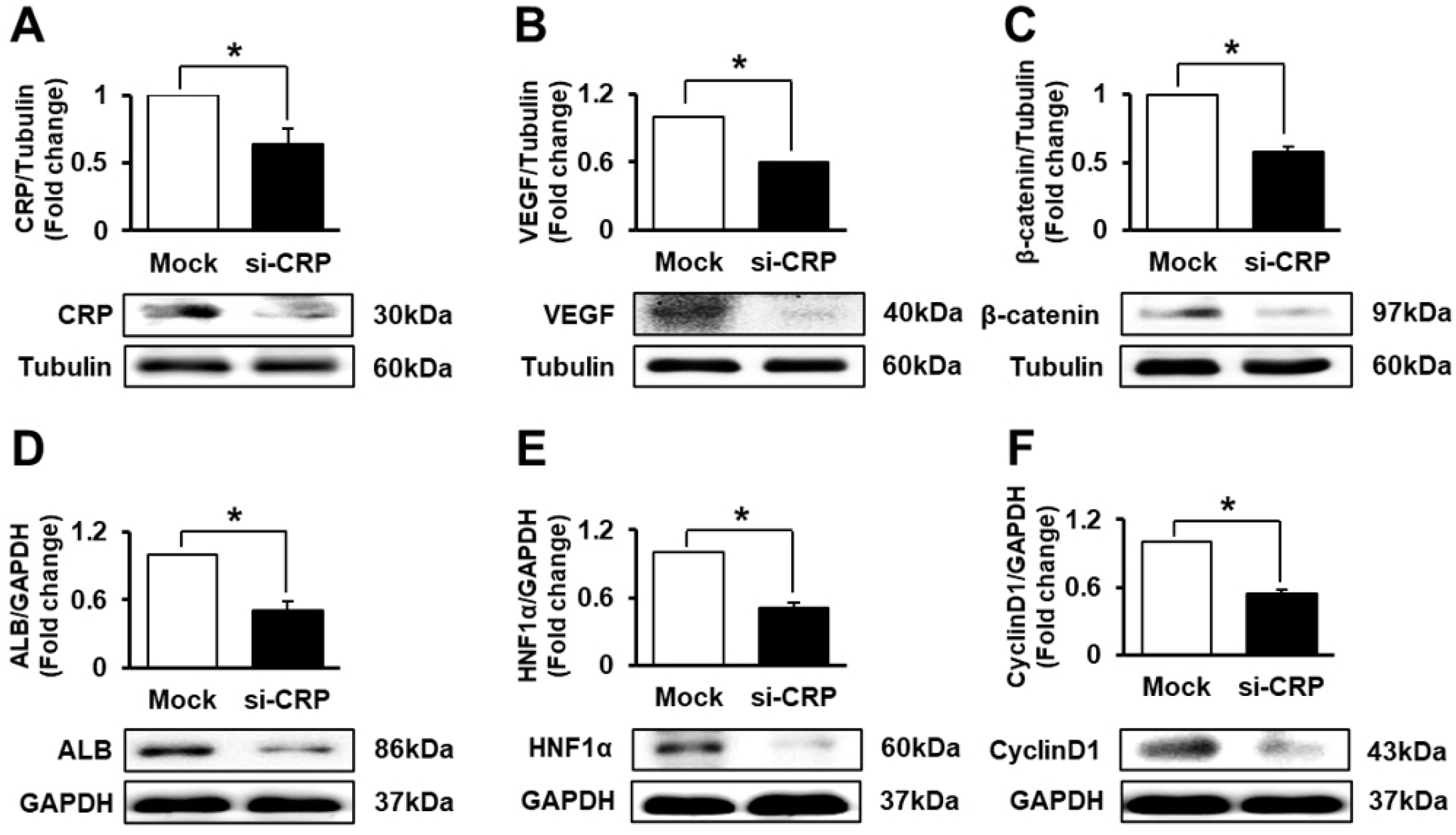
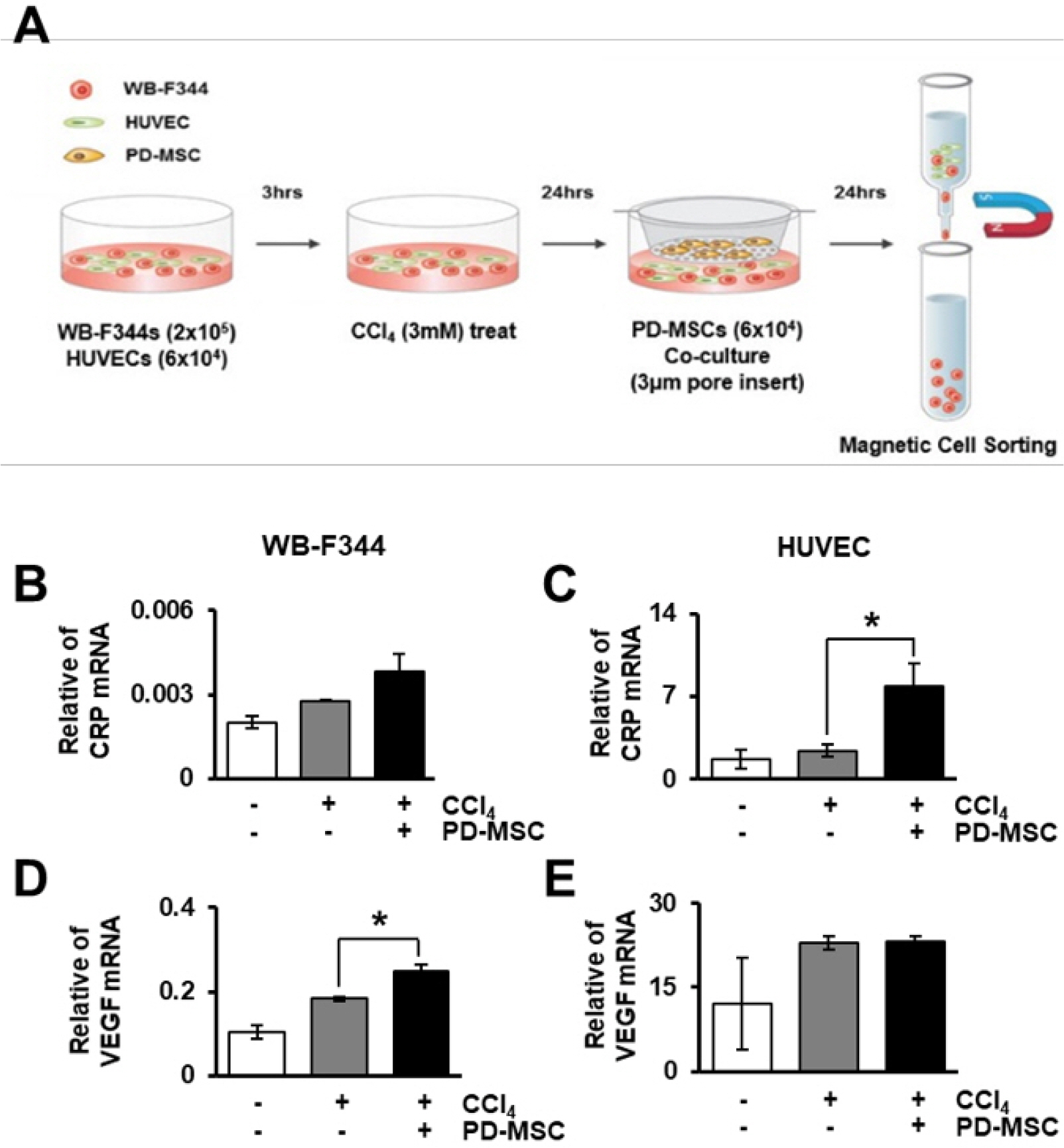
The expression of CRP in CCl 4 -injured rat livers was detected using qRT-PCR and Western blotting after transplantation of placenta-derived mesenchymal stem cells (PD-MSCs) into rats. To determine whether CRP functions in hepatic regeneration by promoting angiogenesis through the Wnt pathway, we detected VEGF and β-catenin in liver tissues and BrdU and β-catenin in hepatocytes by immunofluorescence. The expression levels of CRP, Wnt pathway-related and angiogenic factors were increased in CCl 4 -injured and PD-MSCs transplanted rat livers. In vitro, the expression levels of Wnt signaling and angiogenic factors were decreased in siRNA-CRP-transfected rat hepatocytes.
Conclusions
CRP upregulation by PD-MSCs participates in vascular remodeling to promote liver regeneration via the Wnt signaling pathway during hepatic failure.
Keyword
Figure
Reference
-
References
1. Rui L. 2014; Energy metabolism in the liver. Compr Physiol. 4:177–197. DOI: 10.1002/cphy.c130024. PMID: 24692138. PMCID: PMC4050641.
Article2. Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. 2017; Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 66:212–227. DOI: 10.1016/j.jhep.2016.07.009. PMID: 27423426.
Article3. Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. 2010; Inductive angiocrine signals from sinusoidal endothelium are required for liver regene-ration. Nature. 468:310–315. DOI: 10.1038/nature09493. PMID: 21068842. PMCID: PMC3058628.
Article4. Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. 2014; Divergent angiocrine signals from vascular niche balance liver regene-ration and fibrosis. Nature. 505:97–102. DOI: 10.1038/nature12681. PMID: 24256728. PMCID: PMC4142699.
Article5. Pinzani M, Rosselli M, Zuckermann M. 2011; Liver cirrhosis. Best Pract Res Clin Gastroenterol. 25:281–290. DOI: 10.1016/j.bpg.2011.02.009. PMID: 21497745.
Article6. Salazar J, Martínez MS, Chávez-Castillo M, Núñez V, Añez R, Torres Y, Toledo A, Chacín M, Silva C, Pacheco E, Rojas J, Bermúdez V. 2014; C-reactive protein: an in-depth look into structure, function, and regulation. Int Sch Res Notices. 2014:653045. DOI: 10.1155/2014/653045. PMID: 27433484. PMCID: PMC4897210.
Article7. Eisenhardt SU, Habersberger J, Murphy A, Chen YC, Woollard KJ, Bassler N, Qian H, von Zur Muhlen C, Hagemeyer CE, Ahrens I, Chin-Dusting J, Bobik A, Peter K. 2009; Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ Res. 105:128–137. DOI: 10.1161/CIRCRESAHA.108.190611. PMID: 19520972.
Article8. Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K. 2009; C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 8:3885–3892. DOI: 10.4161/cc.8.23.10068. PMID: 19887916.
Article9. Peña E, de la Torre R, Arderiu G, Slevin M, Badimon L. 2017; mCRP triggers angiogenesis by inducing F3 transcription and TF signalling in microvascular endothelial cells. Thromb Haemost. 117:357–370. DOI: 10.1160/TH16-07-0524. PMID: 27808345.
Article10. Krayem I, Bazzi S, Karam M. 2017; The combination of CRP isoforms with oxLDL decreases TNF-α and IL-6 release by U937-derived macrophages. Biomed Rep. 7:272–276. DOI: 10.3892/br.2017.949. PMID: 28808571. PMCID: PMC5543421.
Article11. Clevers H. 2006; Wnt/beta-catenin signaling in development and disease. Cell. 127:469–480. DOI: 10.1016/j.cell.2006.10.018. PMID: 17081971.12. Thompson MD, Monga SP. 2007; WNT/beta-catenin signaling in liver health and disease. Hepatology. 45:1298–1305. DOI: 10.1002/hep.21651. PMID: 17464972.13. Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. 2014; Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development. 141:1757–1766. DOI: 10.1242/dev.104422. PMID: 24715464.
Article14. Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Göthert JR, Malik AB, Valyi-Nagy T, Zhao YY. 2016; Endothelial β-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation. 133:177–186. DOI: 10.1161/CIRCULATIONAHA.115.015982. PMID: 26538583. PMCID: PMC4814374.
Article15. Lee HJ, Jung J, Cho KJ, Lee CK, Hwang SG, Kim GJ. 2012; Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation. 84:223–231. DOI: 10.1016/j.diff.2012.05.007. PMID: 22885322.
Article16. Zhang D, Jiang M, Miao D. 2011; Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 6:e16789. DOI: 10.1371/journal.pone.0016789. PMID: 21326862. PMCID: PMC3033905.
Article17. Jung J, Moon JW, Choi JH, Lee YW, Park SH, Kim GJ. 2015; Epigenetic alterations of IL-6/STAT3 signaling by placental stem cells promote hepatic regeneration in a rat model with CCl4-induced liver injury. Int J Stem Cells. 8:79–89. DOI: 10.15283/ijsc.2015.8.1.79. PMID: 26019757. PMCID: PMC4445712.
Article18. Jun JH, Choi JH, Bae SH, Oh SH, Kim GJ. 2016; Decreased C-reactive protein induces abnormal vascular structure in a rat model of liver dysfunction induced by bile duct ligation. Clin Mol Hepatol. 22:372–381. DOI: 10.3350/cmh.2016.0032. PMID: 27729629. PMCID: PMC5066379.
Article19. Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, Hultenby K, Christensson B, Ericzon BG, Holgersson J, Sumitran-Holgersson S. 2003; Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 163:1275–1289. DOI: 10.1016/S0002-9440(10)63487-6. PMID: 14507637. PMCID: PMC1868294.
Article20. Iredale JP. 2007; Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 117:539–548. DOI: 10.1172/JCI30542. PMID: 17332881. PMCID: PMC1804370.
Article21. Djonov V, Baum O, Burri PH. 2003; Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 314:107–117. DOI: 10.1007/s00441-003-0784-3. PMID: 14574551.
Article22. Park S, Kim JW, Kim JH, Lim CW, Kim B. 2015; Differential roles of angiogenesis in the induction of fibrogenesis and the resolution of fibrosis in liver. Biol Pharm Bull. 38:980–985. DOI: 10.1248/bpb.b15-00325. PMID: 26133707.
Article23. Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. 2014; β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 60:964–976. DOI: 10.1002/hep.27082. PMID: 24700412. PMCID: PMC4139486.
Article24. Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. 2018; Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: a sequel to the Wnt-Wnt situation. Hepatol Commun. 2:845–860. DOI: 10.1002/hep4.1196. PMID: 30027142. PMCID: PMC6049069.
Article25. Leibing T, Géraud C, Augustin I, Boutros M, Augustin HG, Okun JG, Langhans CD, Zierow J, Wohlfeil SA, Olsavszky V, Schledzewski K, Goerdt S, Koch PS. 2018; Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice. Hepatology. 68:707–722. DOI: 10.1002/hep.29613. PMID: 29059455. PMCID: PMC6099291.
Article26. Oishi N, Yamashita T, Kaneko S. 2014; Molecular biology of liver cancer stem cells. Liver Cancer. 3:71–84. DOI: 10.1159/000343863. PMID: 24944998. PMCID: PMC4057789.
Article27. Moutachakkir M, Lamrani Hanchi A, Baraou A, Boukhira A, Chellak S. 2017; Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann Biol Clin (Paris). 75:225–229. DOI: 10.1684/abc.2017.1232. PMID: 28377336.
Article28. Su HX, Zhou HH, Wang MY, Cheng J, Zhang SC, Hui F, Chen XZ, Liu SH, Liu QJ, Zhu ZJ, Hu QR, Wu Y, Ji SR. 2014; Mutations of C-reactive protein (CRP) -286 SNP, APC and p53 in colorectal cancer: implication for a CRP-Wnt crosstalk. PLoS One. 9:e102418. DOI: 10.1371/journal.pone.0102418. PMID: 25025473. PMCID: PMC4099363.
Article29. Chen J, Gu Z, Wu M, Yang Y, Zhang J, Ou J, Zuo Z, Wang J, Chen Y. 2016; C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1α via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res Ther. 7:114. DOI: 10.1186/s13287-016-0377-1. PMID: 27526687. PMCID: PMC4986362.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- L-Theanine-Treated Adipose-Derived Mesenchymal Stem Cells Alleviate the Cytotoxicity Induced by N-Nitrosodiethylamine in Liver
- Epigenetic Alterations of IL-6/STAT3 Signaling by Placental Stem Cells Promote Hepatic Regeneration in a Rat Model with CCl4-induced Liver Injury
- Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- Mesenchymal Stem Cell Therapy in Pulmonary Disease