J Liver Cancer.
2020 Sep;20(2):128-134. 10.17998/jlc.20.2.128.
Infiltration of T Cells and Programmed Cell Death Ligand 1-expressing Macrophages as a Potential Predictor of Lenvatinib Response in Hepatocellular Carcinoma
- Affiliations
-
- 1The Catholic University Liver Research Center, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Gastroenterology and Hepatology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Gastroenterology and Hepatology, Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2508024
- DOI: http://doi.org/10.17998/jlc.20.2.128
Abstract
- Background/Aims
Lenvatinib was recently proven to be non-inferior to sorafenib in treating unresectable hepatocellular carcinoma (HCC) in a phase-3 randomized controlled trial. In this study, we investigated whether the response to lenvatinib was affected by tumor immunogenicity.
Methods
Between May 2019 and April 2020, nine patients with intermediate-to-advanced HCC, who were treated with lenvatinib after liver biopsy, were enrolled. Immunohistochemical staining and multi-color flow cytometry were performed on specimens obtained from liver biopsy.
Results
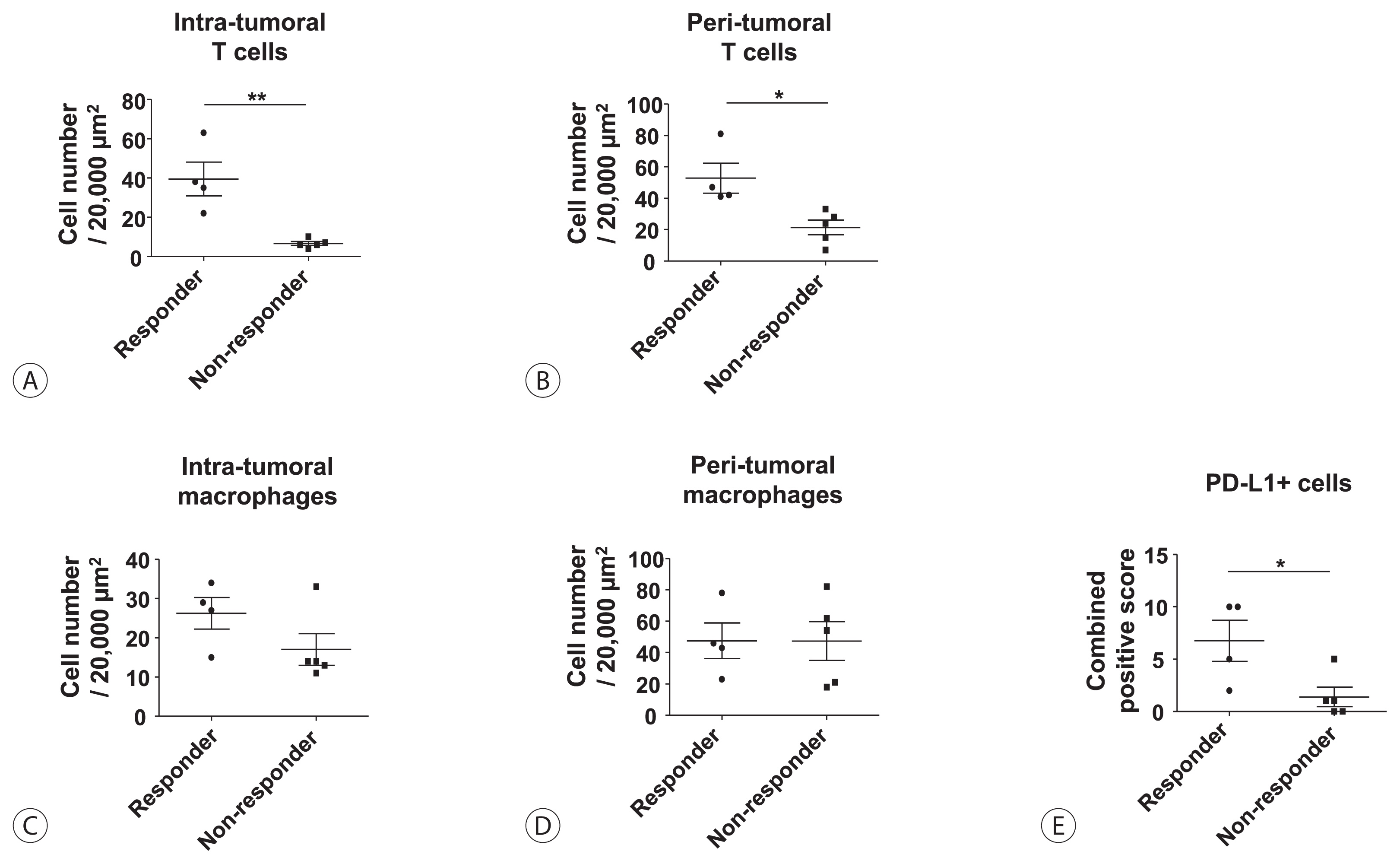
Among the nine patients enrolled, four showed objective responses (complete responses+partial responses). Immunohistochemical staining for CD3, CD68, and programmed cell death ligand 1 (PD-L1) demonstrated that patients with objective responses showed marked infiltration of T cells and PD-L1-expressing macrophages in intra-tumoral and peri-tumoral tissues compared to those without objective responses. A significant difference in the numbers of infiltrated T cells, both in the intra-tumoral (P<0.01) and peri-tumoral regions (P<0.05), were identified between responders and non-responders. Regarding the number of infiltrated macrophages, no significant difference was found between the responders and non-responders, although the number of PD-L1-expressing tumor-associated macrophages was significantly higher in responders than that in non-responders (P<0.05).
Conclusions
Tumor immunogenicity, as indicated by T cell and PD-L1-positive macrophage infiltration, affects lenvatinib response in unresectable HCC.
Keyword
Figure
Reference
-
1. Yoon SK. Molecular mechanism of hepatocellular carcinoma. Hepatoma Research. 2018; 4:42.2. Dika IE, Abou-Alfa GK. Treatment options after sorafenib failure in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2017; 23:273–279.3. Sung PS, Park HL, Yang K, Hwang S, Song MJ, Jang JW, et al. (18) F-fluorodeoxyglucose uptake of hepatocellular carcinoma as a prognostic predictor in patients with sorafenib treatment. Eur J Nucl Med Mol Imaging. 2018; 45:384–391.4. Bangaru S, Marrero JA, Singal AG. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2020; 51:78–89.5. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet. 2018; 391:1163–1173.6. Finn RS, Zhu AX. Evolution of systemic therapy for hepatocellular carcinoma. Hepatology. 2020; May. 7. DOI: 10.1002/hep.31306. [Epub ahead of print].7. Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Sorafenib vs. lenvatinib as first-line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticancer Res. 2020; 40:2283–2290.8. Sung PS, Jang JW. Natural killer cell dysfunction in hepatocellular carcinoma: pathogenesis and clinical implications. Int J Mol Sci. 2018; 19:3648.9. Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019; 7:267.10. Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, et al. A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine. 2017; 22:58–67.11. Ding W, Tan Y, Qian Y, Xue W, Wang Y, Jiang P, et al. Clinicopathologic and prognostic significance of tumor-associated macrophages in patients with hepatocellular carcinoma: a meta-analysis. PLoS One. 2019; 14:e0223971.12. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020; 38:193–202.13. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018; 19:940–952.14. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–2502.15. Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019; 11:1758835919862692.16. Hilmi M, Neuzillet C, Calderaro J, Lafdil F, Pawlotsky JM, Rousseau B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J Immunother Cancer. 2019; 7:333.17. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1–6 hepatocellular carcinoma model. Cancer Sci. 2018; 109:3993–4002.18. Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019; 25:245–263.19. European Association for the Study of the Liver, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.20. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020; 72:288–306.21. Park DJ, Sung PS, Kim JH, Lee GW, Jang JW, Jung ES, et al. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J Immunother Cancer. 2020; 8:e000301.22. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019; 14:e0212513.23. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015; 212:139–148.24. Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee H, et al. VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol. 2019; 4:eaay0555.25. Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-associated macrophages: recent insights and therapies. Front Oncol. 2020; 10:188.26. Degroote H, Van Dierendonck A, Geerts A, Van Vlierberghe H, Devisscher L. Preclinical and clinical therapeutic strategies affecting tumor-associated macrophages in hepatocellular carcinoma. J Immunol Res. 2018; 2018:7819520.27. Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O’Brien S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med. 2019; 11:eaat1500.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exploring the mythical abscopal effect: Radiation and programmed cell death protein 1 (PD-1) blockade for hepatocellular carcinoma
- Kupffer Cells in Hepatocellular Carcinoma
- The role of lenvatinib in the era of immunotherapy of hepatocellular carcinoma
- An update on immunotherapy with PD-1 and PD-L1 blockade
- Radiation-induced abscopal effect and its enhancement by programmed cell death 1 blockade in the hepatocellular carcinoma: A murine model study