Cancer Res Treat.
2020 Oct;52(4):1135-1144. 10.4143/crt.2020.218.
A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair–Deficient/Microsatellite Instability–High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 3Division of Medical Oncology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 5Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 6Division of Oncology/Hematology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 7Division of Hematology–Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 8Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2507939
- DOI: http://doi.org/10.4143/crt.2020.218
Abstract
- Purpose
We evaluated the efficacy and safety of avelumab, an anti-PD-L1 antibody, in patients with metastatic or unresectable colorectal cancer (mCRC) with mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H) or POLE mutations.
Materials and Methods
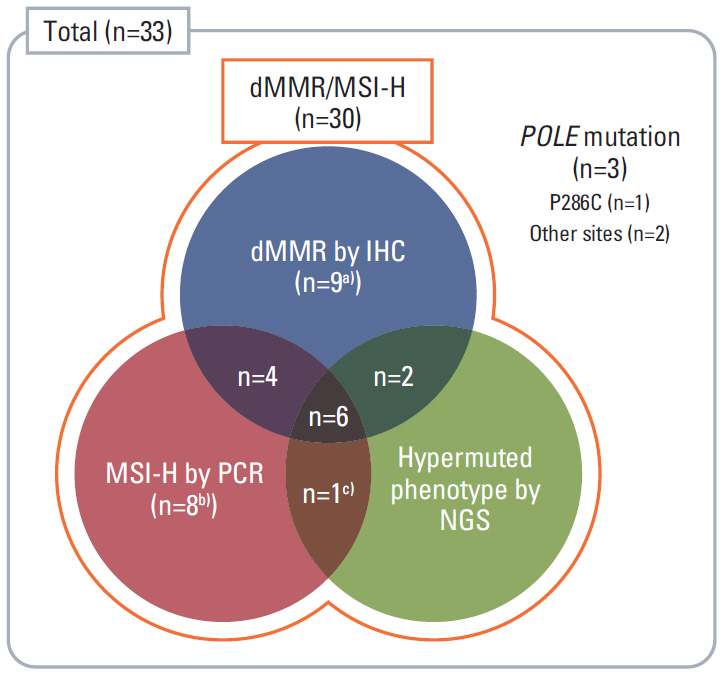
In this prospective, open-label, multicenter phase II study, 33 patients with mCRC harboring dMMR/MSI-H or POLE mutations after failure of ≥1st-line chemotherapy received avelumab 10 mg/kg every 2 weeks. dMMR/MSI-H was confirmed with immunohistochemical staining (IHC) by loss of expression of MMR proteins or polymerase chain reaction (PCR) for microsatellite sequences. POLE mutation was confirmed by next-generation sequencing (NGS). The primary endpoint was the objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors ver. 1.1.
Results
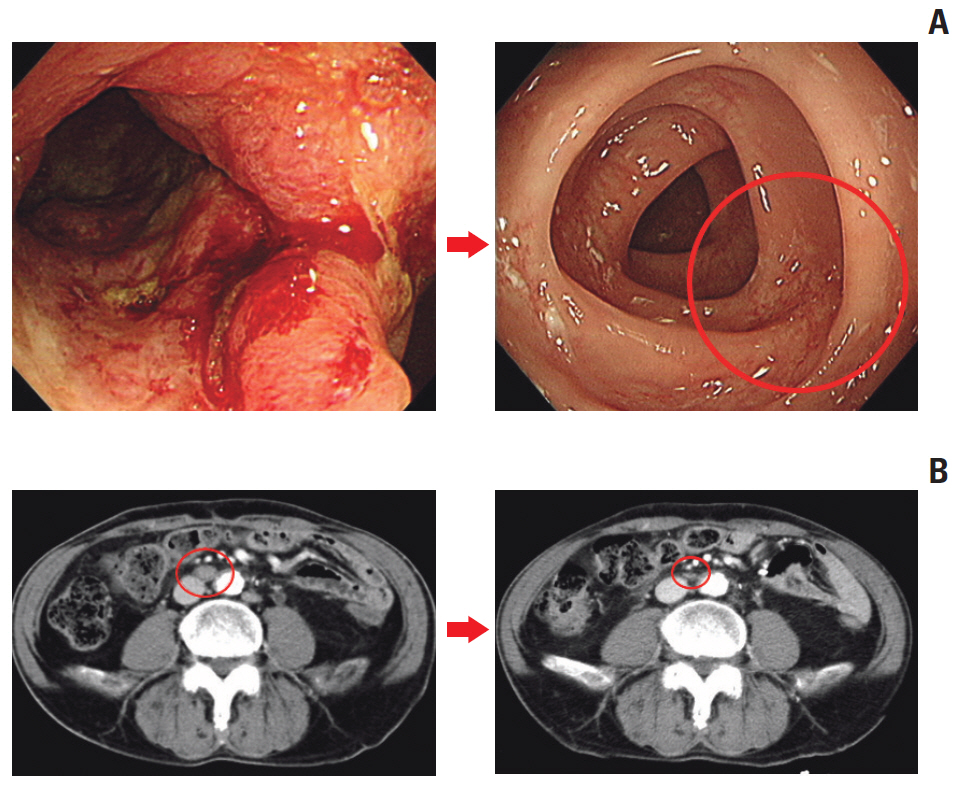
The median age was 60 years, and 78.8% were male. Thirty patients were dMMR/MSI-H and three had POLE mutations. The ORR was 24.2%, and all of the responders were dMMR/MSI-H. For 21 patients with MSI-H by PCR or NGS, the ORR was 28.6%. At a median follow-up duration of 16.3 months, median progression-free survival and overall survival were 3.9 and 13.2 months in all patients, and 8.1 months and not reached, respectively, in patients with MSI-H by PCR or NGS. Dose interruption and discontinuation due to treatment-related adverse events occurred in 4 and 2 patients, respectively, with no treatment-related deaths.
Conclusion
Avelumab displayed antitumor activity with manageable toxicity in patients with previously treated mCRC harboring dMMR/MSI-H. Diagnosis of dMMR/MSI-H with PCR or NGS could be complementary to IHC to select patients who would benefit from immunotherapy.
Keyword
Figure
Reference
-
References
1. Jung KW, Won YJ, Kong HJ, Lee ES; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018; 50:303–16.
Article2. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381:303–12.
Article3. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015; 372:1909–19.
Article4. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–20.5. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017; 18:1182–91.
Article6. Le DT, Kavan P, Kim TW, Burge ME, Van Cutsem E, Hara H, et al. KEYNOTE-164: Pembrolizumab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer. J Clin Oncol. 2018; 36(15 Suppl):3514.
Article7. Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020; 38:11–9.
Article8. Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015; 5:43–51.
Article9. Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009; 100:266–73.
Article10. Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013; 45:136–44.11. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016; 17:1374–85.
Article12. Vaishampayan U, Schoffski P, Ravaud A, Borel C, Peguero J, Chaves J, et al. Avelumab monotherapy as first-line or second-line treatment in patients with metastatic renal cell carcinoma: phase Ib results from the JAVELIN Solid Tumor trial. J Immunother Cancer. 2019; 7:275.
Article13. Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018; 19:51–64.
Article14. Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019; 5:393–401.15. Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJ, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017; 18:599–610.
Article16. Choi YJ, Kim YH. The cancer precision medicine diagnosis and treatment (K-MASTER) enterprise. Korean J Med. 2019; 94:246–51.
Article17. Kelly K, Infante JR, Taylor MH, Patel MR, Wong DJ, Iannotti N, et al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018; 124:2010–7.
Article18. Heery CR, O'Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017; 18:587–98.
Article19. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018; 4:e180013.20. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020; 38:1–10.
Article21. Kelderman S, Schumacher TN, Kvistborg P. Mismatch repairdeficient cancers are targets for anti-PD-1 therapy. Cancer Cell. 2015; 28:11–3.
Article22. Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019; 30:1096–103.
Article23. Mandal R, Samstein RM, Lee KW, Havel JJ, Wang H, Krishna C, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science. 2019; 364:485–91.
Article24. Grasso CS, Giannakis M, Wells DK, Hamada T, Mu XJ, Quist M, et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018; 8:730–49.25. Cohen R, Hain E, Buhard O, Guilloux A, Bardier A, Kaci R, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol. 2019; 5:551–5.
Article26. Klarskov L, Ladelund S, Holck S, Roenlund K, Lindebjerg J, Elebro J, et al. Interobserver variability in the evaluation of mismatch repair protein immunostaining. Hum Pathol. 2010; 41:1387–96.
Article27. Konstantinopoulos PA, Luo W, Liu JF, Gulhan DC, Krasner C, Ishizuka JJ, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019; 37:2786–94.
Article28. Gong J, Wang C, Lee PP, Chu P, Fakih M. Response to PD-1 blockade in microsatellite stable metastatic colorectal cancer harboring a POLE mutation. J Natl Compr Canc Netw. 2017; 15:142–7.29. Sorscher S, Resnick J, Goodman M. First case report of a dramatic radiographic response to a checkpoint inhibitor in a patient with proficient mismatch repair gene expressing metastatic colorectal cancer. JCO Precis Oncol. 2017; Feb. 23. [Epub]. https://doi.org/10.1200/PO.16.00005.
Article30. Goodman AM, Piccioni D, Kato S, Boichard A, Wang HY, Frampton G, et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 2018; 4:1237–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hereditary Nonpolyposis Colorectal Cancer
- Later lines in pMMR/MSS metastatic colorectal cancer: News opportunities with immunotherapy and local treatments
- Immune check point inhibitors in BRAF mutated metastatic colorectal cancer: A review
- Gastric Cancer and DNA Mismatch Repair
- Microsatellite Instability in Colorectal Carcinomas