Cancer Res Treat.
2020 Oct;52(4):1112-1119. 10.4143/crt.2020.245.
Real-World Experience of Nivolumab in Non-small Cell Lung Cancer in Korea
- Affiliations
-
- 1Division of Medical Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Oncology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea
- 4Division of Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 6Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 7Center for Lung Cancer, National Cancer Center, Goyang, Korea
- 8Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 9Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea
- 10Department of Hematology/Oncology, Keimyung University Dongsan Hospital, Daegu, Korea
- 11Division of Medical Oncology, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 12Division of Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 13Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea
- 14Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, Korea
- 15Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Bundang, Korea
- 16Department of Hematology-Oncology, Inje University Haeundae Paik Hospital, Busan, Korea
- 17Division of Hematology/Oncology, Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 18Department of Internal Medicine, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 19Division of Hematology-Oncology, Department of Internal Medicine, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- KMID: 2507937
- DOI: http://doi.org/10.4143/crt.2020.245
Abstract
- Purpose
The introduction of immune checkpoint inhibitors represents a major advance in the treatment of lung cancer, allowing sustained recovery in a significant proportion of patients. Nivolumab is a monoclonal anti–programmed death cell protein 1 antibody licensed for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) after prior chemotherapy. In this study, we describe the demographic and clinical outcomes of patients with advanced NSCLC treated with nivolumab in the Korean expanded access program.
Materials and Methods
Previously treated patients with advanced non-squamous and squamous NSCLC patients received nivolumab at 3 mg/kg every 2 weeks up to 36 months. Efficacy data including investigator-assessed tumor response, progression data, survival, and safety data were collected.
Results
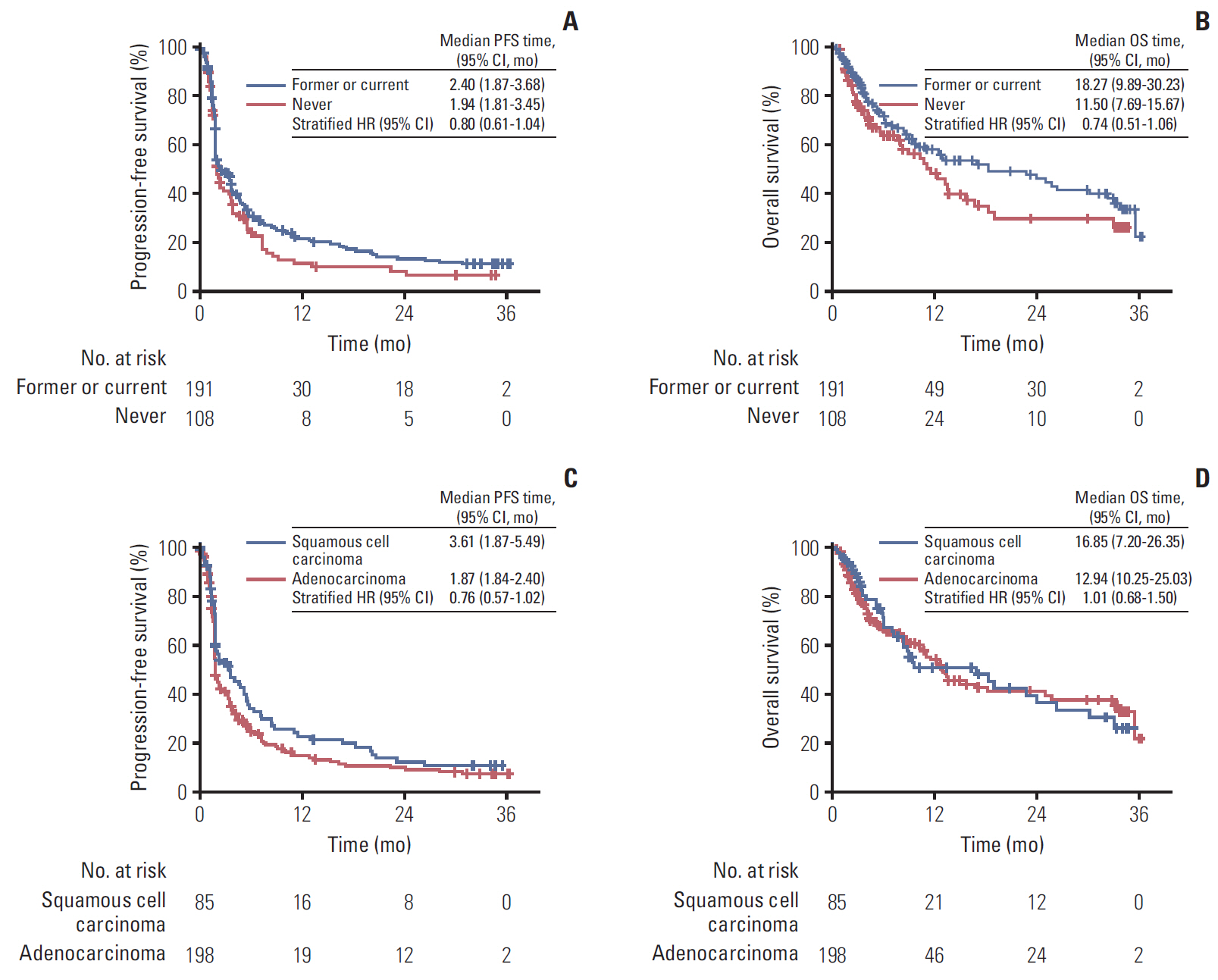
Two hundred ninety-nine patients were treated across 36 Korean centers. The objective response rate and disease control rate were 18% and 49%, respectively; the median progression-free survival was 2.1 months (95% confidence interval [CI], 1.87 to 3.45), and the overall survival (OS) was 13.2 months (95% CI, 10.6 to 18.9). Patients with smoking history and patients who experienced immune-related adverse events showed a prolonged OS. Cox regression analysis identified smoking history, presence of immune-related adverse events as positive factors associated with OS, while liver metastasis was a negative factor associated with OS. The safety profile was generally comparable to previously reported data.
Conclusion
This real-world analysis supports the use of nivolumab for pretreated NSCLC patients, including those with an older age.
Figure
Reference
-
References
1. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to antiPD-1 therapy. Clin Cancer Res. 2014; 20:5064–74.
Article2. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–39.
Article3. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–35.
Article4. Gettinger S, Borghaei H, Brahmer J, Chow L, Burgio M, De Castro Carpeno J, et al. Five-year outcomes from the randomized, phase 3 trials CheckMate 017/057: nivolumab vs docetaxel in previously treated NSCLC. In : 2019 World Conference on Lung Cancer; 2019 Sep 7-10; Barcelona, Spain. p. Abstract No. OA14.04.5. Lilenbaum RC, Cashy J, Hensing TA, Young S, Cella D. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol. 2008; 3:125–9.
Article6. Sacco PC, Casaluce F, Sgambato A, Rossi A, Maione P, Palazzolo G, et al. Current challenges of lung cancer care in an aging population. Expert Rev Anticancer Ther. 2015; 15:1419–29.
Article7. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.8. Rogado J, Sanchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Levi A, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019; 109:21–7.
Article9. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018; 115:71–4.
Article10. Fujii T, Colen RR, Bilen MA, Hess KR, Hajjar J, Suarez-Almazor ME, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. 2018; 36:638–46.
Article11. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017; 12:1798–805.12. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018; 4:374–8.
Article13. Shenoy P, Harugeri A. Elderly patients’ participation in clinical trials. Perspect Clin Res. 2015; 6:184–9.
Article14. Grossi F, Crino L, Logroscino A, Canova S, Delmonte A, Melotti B, et al. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: results from the Italian cohort of an expanded access programme. Eur J Cancer. 2018; 100:126–34.
Article15. Assie JB, Cotte F, Levra MG, Calvet C, Jolivel R, Jouaneton B, et al. Nivolumab outcomes in octogenarian patients with advanced non-small cell lung cancer in a French real-world setting. J Thorac Oncol. 2019; 14(10 Suppl):S708.16. Soo RA, Lim SM, Syn NL, Teng R, Soong R, Mok TS, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer. 2018; 115:12–20.
Article17. Sakamoto H, Tanaka H, Shiratori T, Baba K, Ishioka Y, Itoga M, et al. The efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer harboring driver mutations. Mol Clin Oncol. 2019; 10:610–4.
Article18. Li B, Huang X, Fu L. Impact of smoking on efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer patients: a meta-analysis. Onco Targets Ther. 2018; 11:3691–6.
Article19. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with antiPD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017; 5:417–24.
Article20. Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003; 171:6339–43.
Article21. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013; 14:996–1006.
Article22. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer: a meta-analysis. J Thorac Oncol. 2017; 12:403–7.23. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017; 6:e1356145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lichen Planus Developed During the Treatment with Nivolumab in a Patient with Lung Cancer: A Case Report and Literature Review
- Efficacy and adverse events of immune checkpoint inhibitors: evidence from non-small cell lung cancer and gastric cancer in Korea and Japan
- A Case of Nivolumab-induced Cutaneous Toxicity
- Druggable Targets of Squamous Cell Lung Cancer
- Adjuvant Chemotherapy for Completely Resected Non-Small Cell Lung Cancer