Korean J Physiol Pharmacol.
2020 Nov;24(6):481-492. 10.4196/kjpp.2020.24.6.481.
AITC induces MRP1 expression by protecting against CS/CSE-mediated DJ-1 protein degradation via activation of the DJ-1/Nrf2 axis
- Affiliations
-
- 1School of Pharmacy, Anhui University of Chinese Medicine, Hefei, Anhui 230012, P.R. China
- 2Department of Pharmacy, Lu'an People's Hospital Affiliated to Anhui Medical University, Lu’an, Anhui 237016, P.R. China
- 3Laboratory of Cellular and Molecular Biology, Jiangsu Academy of Chinese Medicine, Nanjing, Jiangsu 210028, P.R. China
- 4Anhui Province Key Laboratory of Research & Development of Chinese Medicine, Hefei 230012, P.R. China
- KMID: 2507731
- DOI: http://doi.org/10.4196/kjpp.2020.24.6.481
Abstract
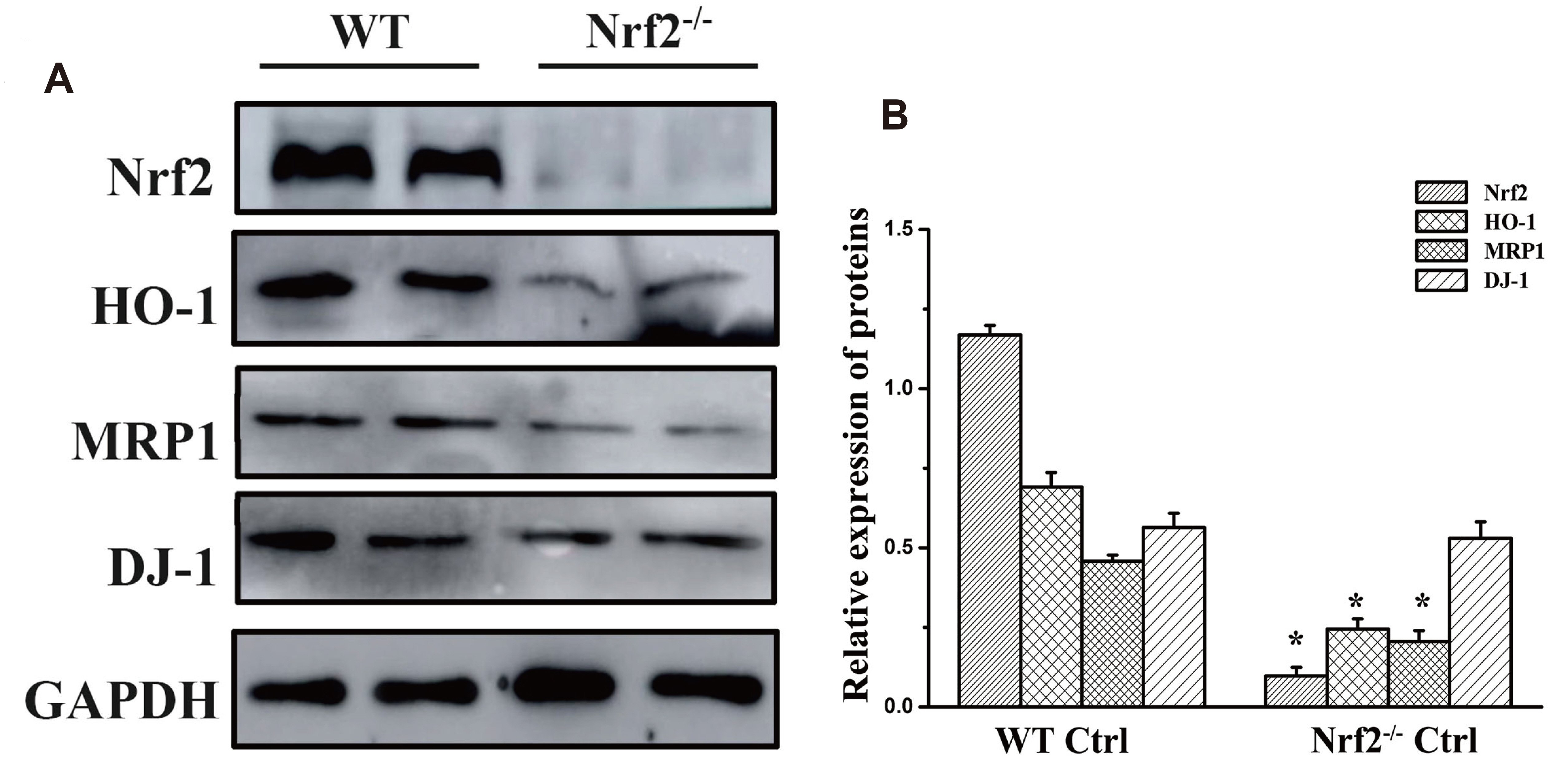
- The present study aimed to examine the effect of allyl isothiocyanate (AITC) on chronic obstructive pulmonary disease and to investigate whether upregulation of multidrug resistance-associated protein 1 (MRP1) associated with the activation of the PARK7 (DJ-1)/nuclear factor erythroid 2-related factor 2 (Nrf2) axis. Lung function indexes and histopathological changes in mice were assessed by lung function detection and H&E staining. The expression levels of Nrf2, MRP1, heme oxygenase-1 (HO-1), and DJ-1 were determined by immunohistochemistry, Western blotting and reverse transcription-quantitative polymerase chain reaction. Next, the expression of DJ-1 in human bronchial epithelial (16HBE) cells was silenced by siRNA, and the effect of DJ-1 expression level on cigarette smoke extract (CSE)-stimulated protein degradation and AITC-induced protein expression was examined. The expression of DJ-1, Nrf2, HO-1, and MRP1 was significantly decreased in the wild type model group, while the expression of each protein was significantly increased after administration of AITC. Silencing the expression of DJ-1 in 16HBE cells accelerated CSE-induced protein degradation, and significantly attenuated the AITC-induced mRNA and protein expression of Nrf2 and MRP1. The present study describes a novel mechanism by which AITC induces MRP1 expression by protecting against CS/CSEmediated DJ-1 protein degradation via activation of the DJ-1/Nrf2 axis.
Keyword
Figure
Cited by 1 articles
-
Metformin alleviates chronic obstructive pulmonary disease and cigarette smoke extract-induced glucocorticoid resistance by activating the nuclear factor E2-related factor 2/heme oxygenase-1 signaling pathway
Fulin Tao, Yuanyuan Zhou, Mengwen Wang, Chongyang Wang, Wentao Zhu, Zhili Han, Nianxia Sun, Dianlei Wang
Korean J Physiol Pharmacol. 2022;26(2):95-111. doi: 10.4196/kjpp.2022.26.2.95.
Reference
-
1. Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. 2006; Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 291:L46–L57. DOI: 10.1152/ajplung.00241.2005. PMID: 16473865.2. Lapperre TS, Postma DS, Gosman MM, Snoeck-Stroband JB, ten Hacken NH, Hiemstra PS, Timens W, Sterk PJ, Mauad T. 2006; Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax. 61:115–121. DOI: 10.1136/thx.2005.040519. PMID: 16055612. PMCID: PMC2104584.
Article3. Cole SP. 2014; Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future. Annu Rev Pharmacol Toxicol. 54:95–117. DOI: 10.1146/annurev-pharmtox-011613-135959. PMID: 24050699.4. van der Deen M, Marks H, Willemse BW, Postma DS, Müller M, Smit EF, Scheffer GL, Scheper RJ, de Vries EG, Timens W. 2006; Diminished expression of multidrug resistance-associated protein 1 (MRP1) in bronchial epithelium of COPD patients. Virchows Arch. 449:682–688. DOI: 10.1007/s00428-006-0240-3. PMID: 17072643.
Article5. Wang DL, Zhang X, Tao XH. 2012; Effects of huatan jiangqi capsule on the levels of multi-drug resistance-associated protein 1 in the bronchial epithelial cells of model rats with chronic obstructive pulmonary disease. Zhongguo Zhong Xi Yi Jie He Za Zhi. 32:955–959. PMID: 23019956.6. van der Deen M, Homan S, Timmer-Bosscha H, Scheper RJ, Timens W, Postma DS, de Vries EG. 2008; Effect of COPD treatments on MRP1-mediated transport in bronchial epithelial cells. Int J Chron Obstruct Pulmon Dis. 3:469–475. DOI: 10.2147/COPD.S2817. PMID: 18990976. PMCID: PMC2629975.7. Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. 1999; Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 47:1541–1548. DOI: 10.1021/jf980985s. PMID: 10564014.8. Munday R, Zhang Y, Fahey JW, Jobson HE, Munday CM, Li J, Stephenson KK. 2006; Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr Cancer. 54:223–231. DOI: 10.1207/s15327914nc5402_9. PMID: 16898867.
Article9. Ye L, Zhang Y. 2001; Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis. 22:1987–1992. DOI: 10.1093/carcin/22.12.1987. PMID: 11751429.
Article10. McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. 2004; Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 134(12 Suppl):3499S–3506S. DOI: 10.1093/jn/134.12.3499S. PMID: 15570060.
Article11. Vomhof-Dekrey EE, Picklo MJ EE Sr. 2012; The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem. 23:1201–1206. DOI: 10.1016/j.jnutbio.2012.03.005. PMID: 22819548.
Article12. Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. 2011; NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 17:363–371. DOI: 10.1016/j.molmed.2011.02.006. PMID: 21459041.
Article13. Lee E, Yin Z, Sidoryk-Węgrzynowicz M, Jiang H, Aschner M. 2012; 15-Deoxy-Δ12,14-prostaglandin J₂ modulates manganese-induced activation of the NF-κB, Nrf2, and PI3K pathways in astrocytes. Free Radic Biol Med. 52:1067–1074. DOI: 10.1016/j.freeradbiomed.2011.12.016. PMID: 22245093.14. Cuevas S, Yang Y, Konkalmatt P, Asico LD, Feranil J, Jones J, Villar VA, Armando I, Jose PA. 2015; Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension. 65:1251–1257. DOI: 10.1161/HYPERTENSIONAHA.114.04525. PMID: 25895590. PMCID: PMC4433423.
Article15. Udasin RG, Wen X, Bircsak KM, Aleksunes LM, Shakarjian MP, Kong AN, Heck DE, Laskin DL, Laskin JD. 2016; Nrf2 regulates the sensitivity of mouse keratinocytes to nitrogen mustard via multidrug resistance-associated protein 1 (Mrp1). Toxicol Sci. 149:202–212. DOI: 10.1093/toxsci/kfv226. PMID: 26454883. PMCID: PMC4715259.
Article16. Wang DL, Wang CY, Cao Y, Zhang X, Tao XH, Yang LL, Chen JP, Wang SS, Li ZG. 2014; Allyl isothiocyanate increases MRP1 function and expression in a human bronchial epithelial cell line. Oxid Med Cell Longev. 2014:547379. DOI: 10.1155/2014/547379. PMID: 24672635. PMCID: PMC3942196.
Article17. Wright JL, Churg A. 2010; Animal models of cigarette smoke-induced chronic obstructive pulmonary disease. Expert Rev Respir Med. 4:723–734. DOI: 10.1586/ers.10.68. PMID: 21128748.
Article18. Cai S, Chen P, Zhang C, Chen JB, Wu J. 2009; Oral N-acetylcysteine attenuates pulmonary emphysema and alveolar septal cell apoptosis in smoking-induced COPD in rats. Respirology. 14:354–359. DOI: 10.1111/j.1440-1843.2009.01511.x. PMID: 19341424.19. Cao Y. 2013. Study on the mechanism of allyl isothiocyanate in the treatment of COPD based on the expression of MRP1 [Master thesis]. Anhui University of Chinese Medicine;Hefei:20. Gueders MM, Bertholet P, Perin F, Rocks N, Maree R, Botta V, Louis R, Foidart JM, Noel A, Evrard B, Cataldo DD. 2008; A novel formulation of inhaled doxycycline reduces allergen-induced inflammation, hyperresponsiveness and remodeling by matrix metalloproteinases and cytokines modulation in a mouse model of asthma. Biochem Pharmacol. 75:514–526. DOI: 10.1016/j.bcp.2007.09.012. PMID: 17950252.
Article21. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. DOI: 10.1006/meth.2001.1262. PMID: 11846609.22. Neuser J, Fraccarollo D, Wick M, Bauersachs J, Widder JD. 2016; Multidrug resistance associated protein-1 (MRP1) deficiency attenuates endothelial dysfunction in diabetes. J Diabetes Complications. 30:623–627. DOI: 10.1016/j.jdiacomp.2016.02.002. PMID: 26908299.
Article23. Gordillo GM, Biswas A, Khanna S, Spieldenner JM, Pan X, Sen CK. 2016; Multidrug resistance-associated protein-1 (MRP-1)-dependent glutathione disulfide (GSSG) efflux as a critical survival factor for oxidant-enriched tumorigenic endothelial cells. J Biol Chem. 291:10089–10103. DOI: 10.1074/jbc.M115.688879. PMID: 26961872. PMCID: PMC4933199.
Article24. Deng J, Coy D, Zhang W, Sunkara M, Morris AJ, Wang C, Chaiswing L, St Clair D, Vore M, Jungsuwadee P. 2015; Elevated glutathione is not sufficient to protect against doxorubicin-induced nuclear damage in heart in multidrug resistance-associated protein 1 (Mrp1/Abcc1) null mice. J Pharmacol Exp Ther. 355:272–279. DOI: 10.1124/jpet.115.225490. PMID: 26354996.
Article25. Yao J, Wei X, Lu Y. 2016; Chaetominine reduces MRP1-mediated drug resistance via inhibiting PI3K/Akt/Nrf2 signaling pathway in K562/Adr human leukemia cells. Biochem Biophys Res Commun. 473:867–873. DOI: 10.1016/j.bbrc.2016.03.141. PMID: 27038543.
Article26. Hong YB, Kang HJ, Kwon SY, Kim HJ, Kwon KY, Cho CH, Lee JM, Kallakury BV, Bae I. 2010; Nuclear factor (erythroid-derived 2)-like 2 regulates drug resistance in pancreatic cancer cells. Pancreas. 39:463–472. DOI: 10.1097/MPA.0b013e3181c31314. PMID: 20118824. PMCID: PMC3506252.
Article27. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. 2002; Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 62:5196–5203. PMID: 12234984.28. Raninga PV, Trapani GD, Tonissen KF. 2014; Cross talk between two antioxidant systems, thioredoxin and DJ-1: consequences for cancer. Oncoscience. 1:95–110. DOI: 10.18632/oncoscience.12. PMID: 25593990. PMCID: PMC4295760.
Article29. Wilson MA. 2011; The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 15:111–122. DOI: 10.1089/ars.2010.3481. PMID: 20812780. PMCID: PMC3110098.
Article30. Zhang XL, Yuan YH, Shao QH, Wang ZZ, Zhu CG, Shi JG, Ma KL, Yan X, Chen NH. 2017; DJ-1 regulating PI3K-Nrf2 signaling plays a significant role in bibenzyl compound 20C-mediated neuroprotection against rotenone-induced oxidative insult. Toxicol Lett. 271:74–83. DOI: 10.1016/j.toxlet.2017.02.022. PMID: 28245986.
Article31. Vavougios G, Zarogiannis SG, Doskas T. 2018; The putative interplay between DJ-1/NRF2 and Dimethyl Fumarate: a potentially important pharmacological target. Mult Scler Relat Disord. 21:88–91. DOI: 10.1016/j.msard.2018.02.027. PMID: 29529529.
Article32. Li R, Wang S, Li T, Wu L, Fang Y, Feng Y, Zhang L, Chen J, Wang X. 2019; Salidroside protects dopaminergic neurons by preserving complex I activity via DJ-1/Nrf2-mediated antioxidant pathway. Parkinsons Dis. 2019:6073496. DOI: 10.1155/2019/6073496. PMID: 31223467. PMCID: PMC6541949.
Article33. Srivastava S, Blower PJ, Aubdool AA, Hider RC, Mann GE, Siow RC. 2016; Cardioprotective effects of Cu(II)ATSM in human vascular smooth muscle cells and cardiomyocytes mediated by Nrf2 and DJ-1. Sci Rep. 6:7. DOI: 10.1038/s41598-016-0012-5. PMID: 28442712. PMCID: PMC5431352.
Article34. Saidu NE, Noé G, Cerles O, Cabel L, Kavian-Tessler N, Chouzenoux S, Bahuaud M, Chéreau C, Nicco C, Leroy K, Borghese B, Goldwasser F, Batteux F, Alexandre J. 2017; Dimethyl fumarate controls the NRF2/DJ-1 axis in cancer cells: therapeutic applications. Mol Cancer Ther. 16:529–539. DOI: 10.1158/1535-7163.MCT-16-0405. PMID: 28069874.
Article35. Bahmed K, Messier EM, Zhou W, Tuder RM, Freed CR, Chu HW, Kelsen SG, Bowler RP, Mason RJ, Kosmider B. 2016; DJ-1 modulates nuclear erythroid 2-related factor-2-mediated protection in human primary alveolar type II cells in smokers. Am J Respir Cell Mol Biol. 55:439–449. DOI: 10.1165/rcmb.2015-0304OC. PMID: 27093578. PMCID: PMC5023027.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metformin alleviates chronic obstructive pulmonary disease and cigarette smoke extract-induced glucocorticoid resistance by activating the nuclear factor E2-related factor 2/heme oxygenase-1 signaling pathway
- Fraxetin Induces Heme Oxygenase-1 Expression by Activation of Akt/Nrf2 or AMP-activated Protein Kinase α/Nrf2 Pathway in HaCaT Cells
- Impact of Nrf2 overexpression on cholangiocarcinoma treatment and clinical prognosis
- Sumoylation of Hes6 Regulates Protein Degradation and Hes1-Mediated Transcription
- Dysregulation of NRF2 in Cancer: from Molecular Mechanisms to Therapeutic Opportunities