Anat Cell Biol.
2020 Sep;53(3):342-354. 10.5115/acb.20.099.
Inhibitory potentials of Cymbopogon citratus oil against aluminium-induced behavioral deficits and neuropathology in rats
- Affiliations
-
- 1Division of Neurobiology, Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, University of Ilorin, Ilorin, Nigeria
- 2Division of Neurobiology, Department of Anatomy, Faculty of Basic Medical Sciences, Adeleke University, Ede, Nigeria
- 3Center for Studies in Behavioral Neurobiology, Department of Psychology, Concordia University Montreal, Montreal, Quebec, Canada
- KMID: 2507647
- DOI: http://doi.org/10.5115/acb.20.099
Abstract
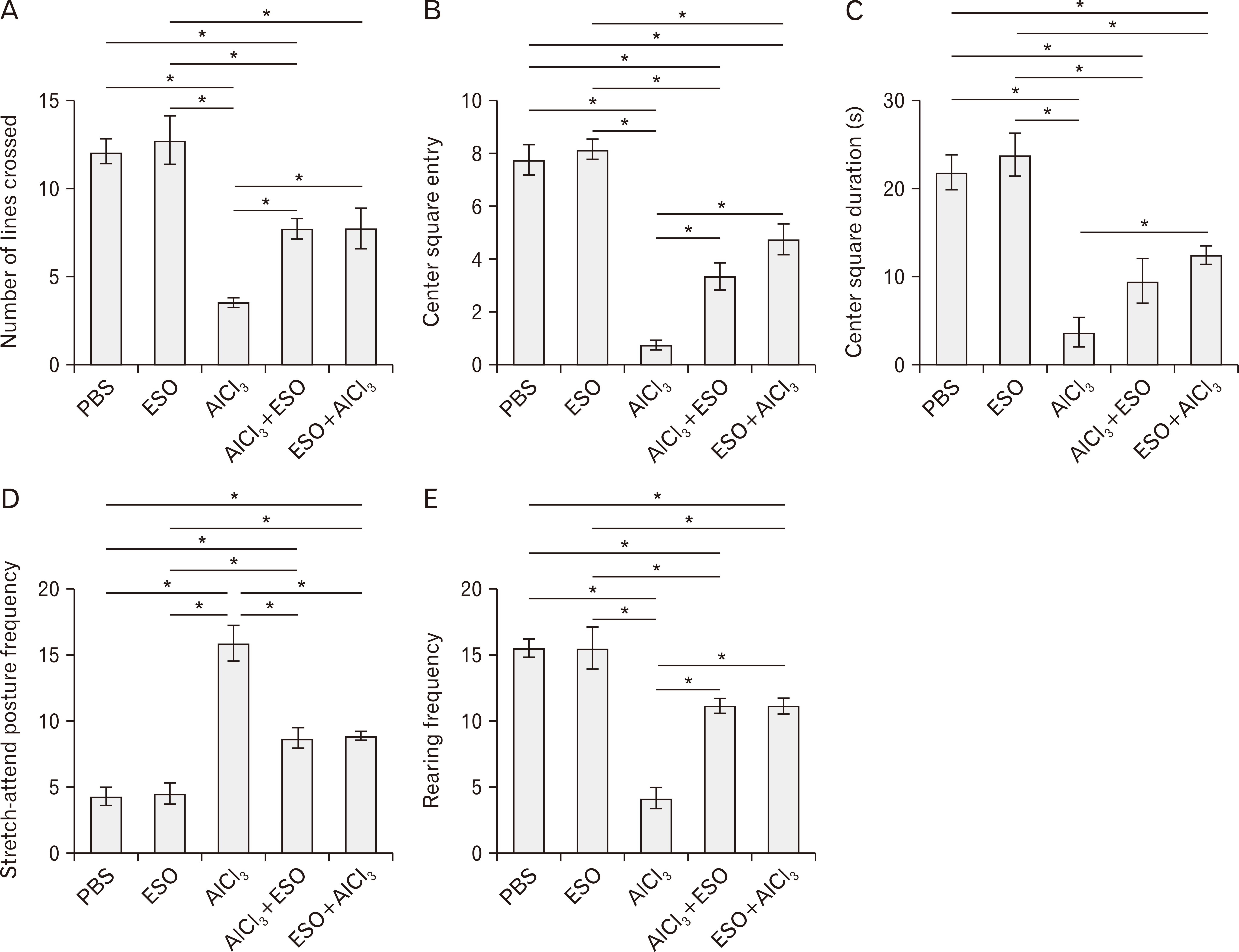
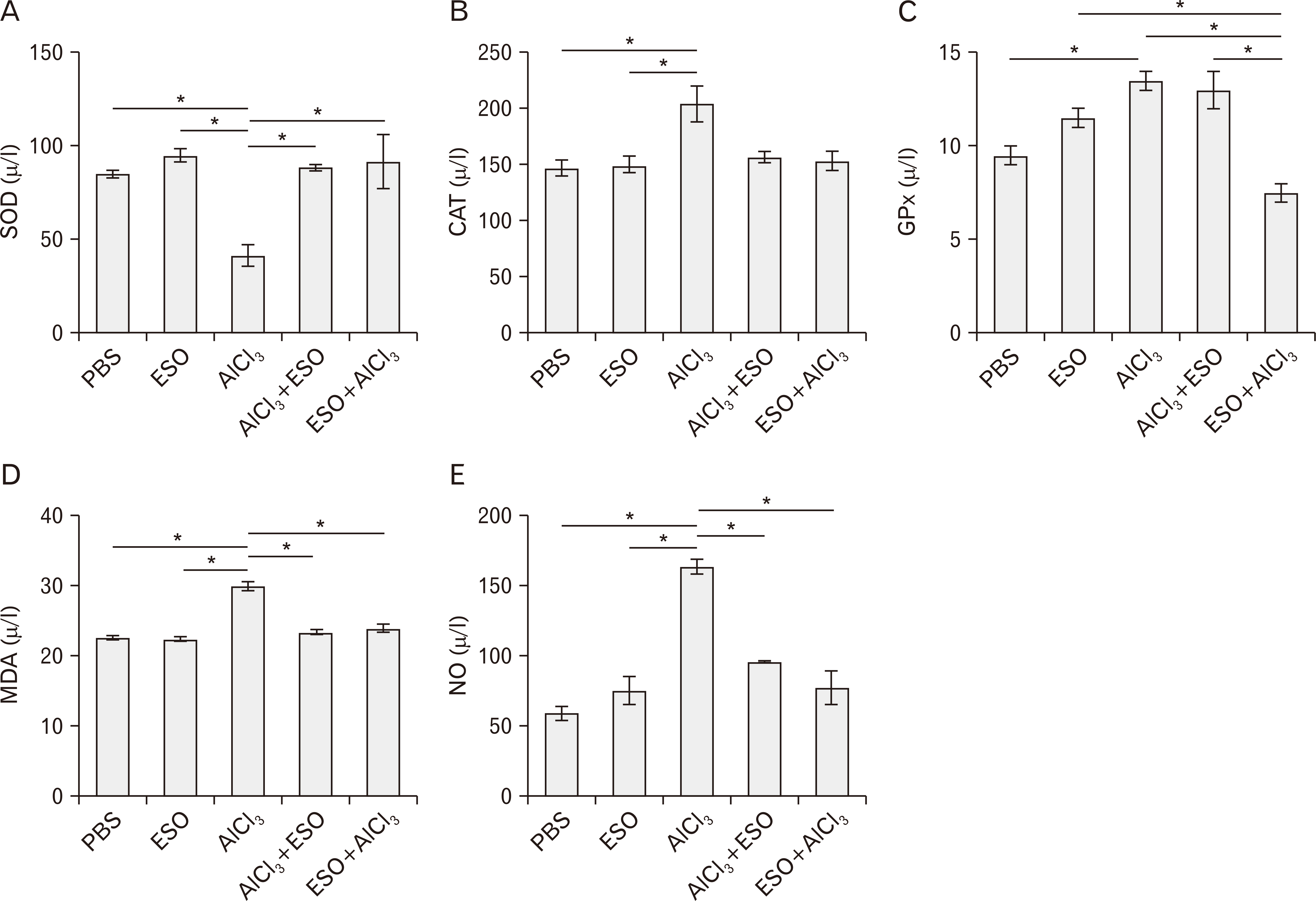
- Cymbopogon citratus is a tropical phytomedicinal plant that is widely known for its hypoglycemic, hypolipidemic, anxiolytic, sedative, antioxidative and anti-inflammatory properties. In this study, we have examined the neuroprotective effects of the essential oil (ESO) of Cymbopogon citratus, following aluminum chloride (AlCl3)-induced neurotoxicity within the cerebellum of Wistar rats. A total of 40 adult male Wistar rats were assigned into five groups and treated orally as follows: A–phosphate-buffered saline (1 ml daily for 15 days); B–ESO (50 mg/kg daily for 15 days); C–AlCl3 (100 mg/kg daily for 15 days); D–AlCl3 then ESO (100 mg/kg AlCl3 daily for 15 days followed by 50 mg/kg ESO daily for subsequent 15 days); E– ESO then AlCl3 (50 mg/kg ESO daily for 15 days followed by 100 mg/kg AlCl3 daily for following 15 days). To address our questions, we observed the locomotion and exploratory behavior of the rats in the open field apparatus and subsequently evaluated cerebellar oxidative redox parameters, neural bioenergetics, acetylcholinesterase levels, transferrin receptor protein, and total protein profiles by biochemical assays. Furthermore, we investigated cerebellar histomorphology and Nissl profile by H&E and Cresyl violet Nissl staining procedures. ESO treatment markedly attenuated deficits in exploratory activities and rearing behavior following AlCl3 toxicity, indicating its anxiolytic potentials. Additionally, AlCl3 evokedincrease in malondialdehyde and nitric oxide levels, as well as repressed cerebellar catalase, glutathione peroxidase, and superoxide dismutase profiles were normalised to baseline levels by ESO treatment. Treatment with ESO, ergo, exhibits substantial neuroprotective and modulatory potentials in response to AlCl3 toxicity.
Keyword
Figure
Reference
-
References
1. Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. 2012; Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 71:362–81. DOI: 10.1097/NEN.0b013e31825018f7. PMID: 22487856. PMCID: PMC3560290.
Article2. Mota SI, Ferreira IL, Rego AC. 2014; Dysfunctional synapse in Alzheimer's disease - a focus on NMDA receptors. Neuropharmacology. 76 Pt A:16–26. DOI: 10.1016/j.neuropharm.2013.08.013. PMID: 23973316. PMCID: PMC7507095.
Article3. Hardy J, Selkoe DJ. 2002; The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 297:353–6. DOI: 10.1126/science.1072994. PMID: 12130773.
Article4. Wu T, Hallett M. 2013; The cerebellum in Parkinson's disease. Brain. 136(Pt 3):696–709. DOI: 10.1093/brain/aws360. PMID: 23404337.
Article5. Olajide OJ, Ugbosanmi AT, Enaibe BU, Ogunrinola KY, Lewu SF, Asogwa NT, Akapa T, Imam A, Ibrahim A, Gbadamosi IT, Yawson EO. 2017; Cerebellar molecular and cellular characterization in rat models of Alzheimer's disease: neuroprotective mechanisms of Garcinia biflavonoid complex. Ann Neurosci. 24:32–45. DOI: 10.1159/000464421. PMID: 28827919.6. Mathiyazahan DB, Thenmozhi AJ, Manivasagam T. 2015; Protective effect of black tea extract against aluminium chloride-induced Alzheimer's disease in rats: a behavioural, biochemical and molecular approach. J Funct Foods. 16:423–35. DOI: 10.1016/j.jff.2015.05.001.
Article7. Rodella LF, Ricci F, Borsani E, Stacchiotti A, Foglio E, Favero G, Rezzani R, Mariani C, Bianchi R. 2008; Aluminium exposure induces Alzheimer's disease-like histopathological alterations in mouse brain. Histol Histopathol. 23:433–9.8. Kumar A, Prakash A, Dogra S. 2011; Neuroprotective effect of carvedilol against aluminium induced toxicity: possible behavioral and biochemical alterations in rats. Pharmacol Rep. 63:915–23. DOI: 10.1016/S1734-1140(11)70607-7. PMID: 22001979.
Article9. Roskams AJ, Connor JR. 1990; Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci U S A. 87:9024–7. DOI: 10.1073/pnas.87.22.9024. PMID: 2247478. PMCID: PMC55093.
Article10. Crapper DR, Krishnan SS, Quittkat S. 1976; Aluminium, neurofibrillary degeneration and Alzheimer's disease. Brain. 99:67–80. DOI: 10.1093/brain/99.1.67. PMID: 963531.
Article11. McDonald B, Esiri MM, Morris J. 1993; Aluminium and Alzheimer's disease. Age Ageing. 22:392–3. DOI: 10.1093/ageing/22.5.392. PMID: 8237634.
Article12. Adeneye AA, Agbaje EO. 2007; Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. J Ethnopharmacol. 112:440–4. DOI: 10.1016/j.jep.2007.03.034. PMID: 17513076.13. Tapia A, Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G, Gerth A, Wilken D, Jordan M, Jiménez-González E, Gomez-Kosky R, Mendoza EQ. 2007; Free radical scavengers from Cymbopogon citratus (DC.) stapf plants cultivated in bioreactors by the temporary immersion (TIS) principle. Z Naturforsch C J Biosci. 62:447–57. DOI: 10.1515/znc-2007-5-620. PMID: 17708453.14. Orrego R, Leiva E, Cheel J. 2009; Inhibitory effect of three C-glycosylflavonoids from Cymbopogon citratus (Lemongrass) on human low density lipoprotein oxidation. Molecules. 14:3906–13. DOI: 10.3390/molecules14103906. PMID: 19924037. PMCID: PMC6254720.15. Campos J, Schmeda-Hirschmann G, Leiva E, Guzmán L, Orrego R, Fernández P, González M, Radojkovic C, Zuñiga FA, Lamperti L, Pastene E, Aguayo C. 2014; Lemon grass (Cymbopogon citratus (D.C) Stapf) polyphenols protect human umbilical vein endothelial cell (HUVECs) from oxidative damage induced by high glucose, hydrogen peroxide and oxidised low-density lipoprotein. Food Chem. 151:175–81. DOI: 10.1016/j.foodchem.2013.11.018. PMID: 24423518.16. Salim E, Kumolosasi E, Jantan I. 2014; Inhibitory effect of selected medicinal plants on the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated human peripheral blood mononuclear cells. J Nat Med. 68:647–53. DOI: 10.1007/s11418-014-0841-0. PMID: 24799081.
Article17. Francisco V, Costa G, Figueirinha A, Marques C, Pereira P, Miguel Neves B, Celeste Lopes M, García-Rodríguez C, Teresa Cruz M, Teresa Batista M. 2013; Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: contribution of chlorogenic acid. J Ethnopharmacol. 148:126–34. DOI: 10.1016/j.jep.2013.03.077. PMID: 23583902.18. Figueirinha A, Paranhos A, Pérez-Alonso JJ, Santos-Buelga C, Batista MT. 2008; Cymbopogon citratus leaves: characterization of flavonoids by HPLC-PDA-ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 110:718–28. DOI: 10.1016/j.foodchem.2008.02.045.19. Garcia R, Ferreira JP, Costa G, Santos T, Branco F, Caramona M, de Carvalho M, Dinis AM, Batista MT, Castel-Branco M, Figueiredo IV. 2015; Evaluation of anti-inflammatory and analgesic activities of cymbopogon citratus in vivo-polyphenols contribution. Res J Med Plant. 9:1–13. DOI: 10.3923/rjmp.2015.1.13.20. Ganjewala D. 2009; Cymbopogon essential oils: chemical compositions and bioactivities. Int J Essent Oil Ther. 3:56–65.21. Stanford SC. 2007; The open field test: reinventing the wheel. J Psychopharmacol. 21:134–5. DOI: 10.1177/0269881107073199. PMID: 17329288.
Article22. Nehru B, Bhalla P. 2007; Biochemical alterations in rat brain following aluminum exposure: protection with centrophenoxine. Toxicol Environ Chem. 89:577–85. DOI: 10.1080/02772240701228763.
Article23. Amjad S, Umesalma S. 2015; Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain histopathological, and biochemical approach. J Mol Biomark Diagn. 6:212. DOI: 10.4172/2155-9929.1000212.
Article24. Lakshmi BV, Sudhakar M, Prakash KS. 2015; Protective effect of selenium against aluminum chloride-induced Alzheimer's disease: behavioral and biochemical alterations in rats. Biol Trace Elem Res. 165:67–74. DOI: 10.1007/s12011-015-0229-3. PMID: 25613582.
Article25. Olajide OJ, Fatoye JO, Idowu OF, Ilekoya D, Gbadamosi IT, Gbadamosi MT, Asogwa NT. 2018; Reversal of behavioral decline and neuropathology by a complex vitamin supplement involves modulation of key neurochemical stressors. Environ Toxicol Pharmacol. 62:120–31. DOI: 10.1016/j.etap.2018.07.005. PMID: 30005307.
Article26. Taïr K, Kharoubi O, Taïr OA, Hellal N, Benyettou I, Aoues A. 2016; Aluminium-induced acute neurotoxicity in rats: treatment with aqueous extract of Arthrophytum (Hammada scoparia). J Acute Disease. 5:470–82. DOI: 10.1016/j.joad.2016.08.028.
Article27. Olajide OJ, Enaibe BU, Bankole OO, Akinola OB, Laoye BJ, Ogundele OM. 2016; Kolaviron was protective against sodium azide (NaN3) induced oxidative stress in the prefrontal cortex. Metab Brain Dis. 31:25–35. DOI: 10.1007/s11011-015-9674-0. PMID: 25916484.
Article28. Kumar V, Gill KD. 2014; Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 41:154–66. DOI: 10.1016/j.neuro.2014.02.004. PMID: 24560992.
Article29. Sayre LM, Perry G, Smith MA. 2008; Oxidative stress and neurotoxicity. Chem Res Toxicol. 21:172–88. DOI: 10.1021/tx700210j. PMID: 18052107.
Article30. Yuan CY, Lee YJ, Hsu GS. 2012; Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J Biomed Sci. 19:51. DOI: 10.1186/1423-0127-19-51. PMID: 22613782. PMCID: PMC3404950.
Article31. Guix FX, Uribesalgo I, Coma M, Muñoz FJ. 2005; The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 76:126–52. DOI: 10.1016/j.pneurobio.2005.06.001. PMID: 16115721.
Article32. Pacher P, Beckman JS, Liaudet L. 2007; Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 87:315–424. DOI: 10.1152/physrev.00029.2006. PMID: 17237348. PMCID: PMC2248324.
Article33. Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. 2007; Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 8:766–75. DOI: 10.1038/nrn2214. PMID: 17882254. PMCID: PMC4903952.
Article34. Chen Z, Zhong C. 2013; Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 108:21–43. DOI: 10.1016/j.pneurobio.2013.06.004. PMID: 23850509.
Article35. Zhang X, Yang F, Xu C, Liu W, Wen S, Xu Y. 2008; Cytotoxicity evaluation of three pairs of hexabromocyclododecane (HBCD) enantiomers on Hep G2 cell. Toxicol In Vitro. 22:1520–7. DOI: 10.1016/j.tiv.2008.05.006. PMID: 18644697.
Article36. Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, Larsson NG, Hoffer BJ, Olson L. 2010; High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci U S A. 107:20087–92. DOI: 10.1073/pnas.1008189107. PMID: 21041631. PMCID: PMC2993405.
Article37. Trapp GA. 1983; Plasma aluminum is bound to transferrin. Life Sci. 33:311–6. DOI: 10.1016/S0024-3205(83)80002-2. PMID: 6410138. PMCID: PMC6804145.
Article38. Al-Hashem F. 2009; Camel's milk protects against aluminum chloride-induced normocytic normocromic anemia, lipid peroxidation and oxidative stress in erythrocytes of white albino rats. Am J Biochem Biotechnol. 5:126–36. DOI: 10.3844/ajbbsp.2009.126.136.
Article39. Goldstein ME, Cooper HS, Bruce J, Carden MJ, Lee VM, Schlaepfer WW. 1987; Phosphorylation of neurofilament proteins and chromatolysis following transection of rat sciatic nerve. J Neurosci. 7:1586–94. DOI: 10.1523/JNEUROSCI.07-05-01586.1987. PMID: 3106591. PMCID: PMC6568824.
Article40. Ekong MB, Ekpo MM, Akpanyung EO, Nwaokonko DU. 2017; Neuroprotective effect of Moringa oleifera leaf extract on aluminium-induced temporal cortical degeneration. Metab Brain Dis. 32:1437–47. DOI: 10.1007/s11011-017-0011-7. PMID: 28397152.
Article41. Khalil EA. 2011; Evaluation of the possible protective and therapeutic influence of coriander (Coriandum sativum L.) seed aqueous extract on hippocampal pyramidal cells against Alzheimer's disease induced by aluminum chloride in adult male albino rats. Researcher. 3:22–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Colorado Potato Beetle (Leptinotarsa decemlineata Say) Control Potential of Essential Oil Isolated from Iranian Cymbopogon citratus Stapf
- Antimicrobial Activity of Some Essential Oils Against Microorganisms Deteriorating Fruit Juices
- Antimicrobial property of lemongrass (Cymbopogon citratus) oil against pathogenic bacteria isolated from pet turtles
- Essential Oil Prepared from Cymbopogon citrates Exerted an Antimicrobial Activity Against Plant Pathogenic and Medical Microorganisms
- Effect of administration of etretinate and fish oil on plasma cholesterol levels in rats