J Korean Neurosurg Soc.
2020 Sep;63(5):579-589. 10.3340/jkns.2019.0182.
An Optimization of AAV-82Q-Delivered Rat Model of Huntington’s Disease
- Affiliations
-
- 1Institute for Stem Cell & Regenerative Medicine (ISCRM), Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea
- 2Laboratory of Veterinary Embryology and Biotechnology (VETEMBIO), Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea
- 3Department of Neurosurgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Medical Neuroscience, College of Medicine, Chungbuk National University, Cheongju, Korea
- 5Department of Neurosurgery, Chungbuk National University Hospital, Cheongju, Korea
- 6Department of Biochemistry and Medical Research Center, Chungbuk National University, Cheongju, Korea
- 7Laboratory of Veterinary Pathology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea
- KMID: 2506021
- DOI: http://doi.org/10.3340/jkns.2019.0182
Abstract
Objective
: No optimum genetic rat Huntington model both neuropathological using an adeno-associated virus (AAV-2) vector vector has been reported to date. We investigated whether direct infection of an AAV2 encoding a fragment of mutant huntingtin (AV2-82Q) into the rat striatum was useful for optimizing the Huntington rat model.
Methods
: We prepared ten unilateral models by injecting AAV2-82Q into the right striatum, as well as ten bilateral models. In each group, five rats were assigned to either the 2×1012 genome copies (GC)/mL of AAV2-82Q (×1, low dose) or 2×1013 GC/mL of AAV2-82Q (×10, high dose) injection model. Ten unilateral and ten bilateral models injected with AAV-empty were also prepared as control groups. We performed cylinder and stepping tests 2, 4, 6, and 8 weeks after injection, tested EM48 positive mutant huntingtin aggregates.
Results
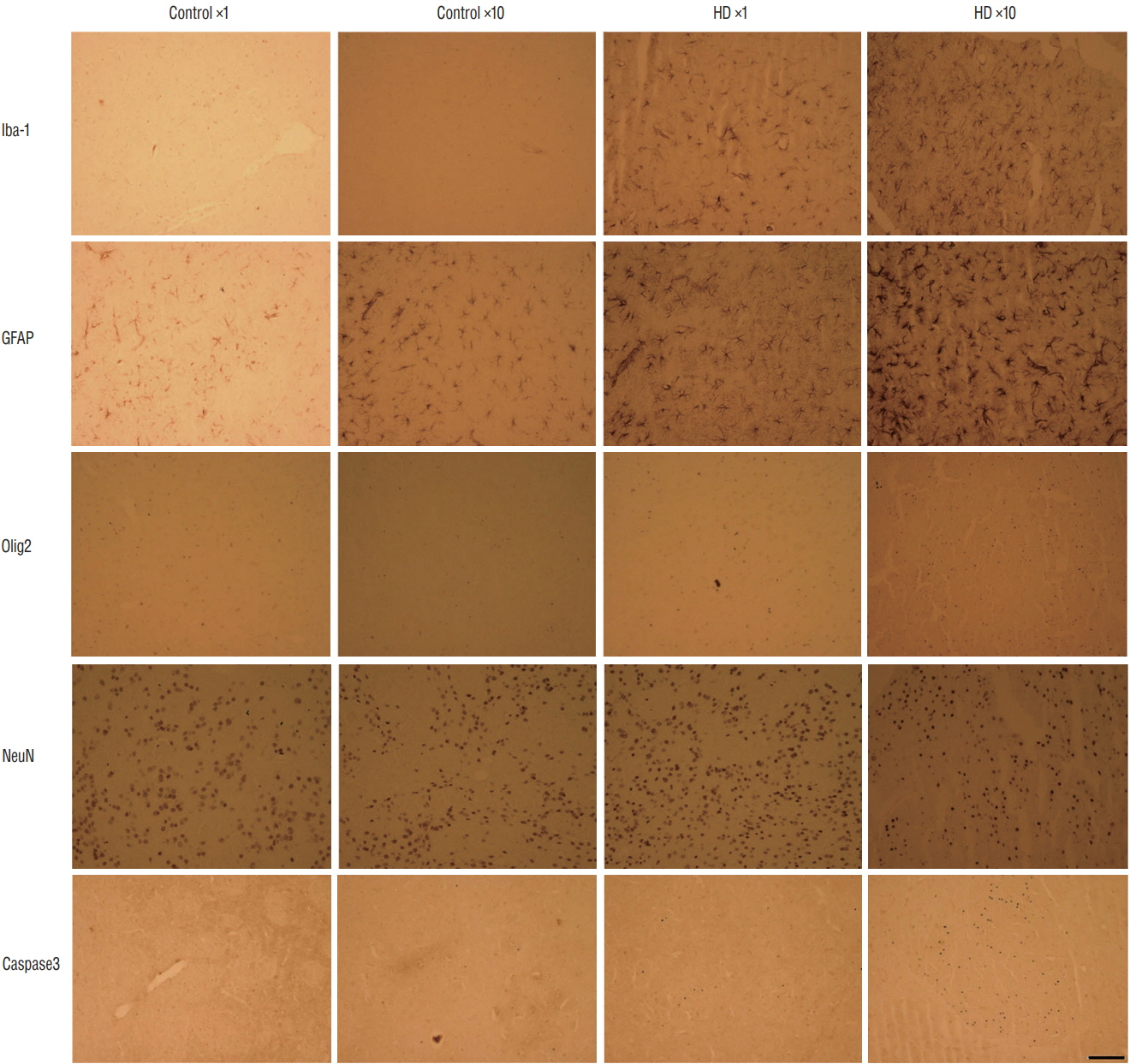
: The high dose of unilateral and bilateral AAV2-82Q model showed a greater decrease in performance on the stepping and cylinder tests. We also observed more prominent EM48-positive mutant huntingtin aggregates in the medium spiny neurons of the high dose of AAV2-82Q injected group.
Conclusion
: Based on the results from the present study, high dose of AAV2-82Q is the optimum titer for establishing a Huntington rat model. Delivery of high dose of human AAV2-82Q resulted in the manifestation of Huntington behaviors and optimum expression of the huntingtin protein in vivo.
Keyword
Figure
Reference
-
References
1. Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 64:133–150. 1990.
Article2. Cattoglio C, Facchini G, Sartori D, Antonelli A, Miccio A, Cassani B, et al. Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood. 110:1770–1778. 2007.
Article3. Ceccarelli I, Fiengo P, Remelli R, Miragliotta V, Rossini L, Biotti I, et al. Recombinant Adeno Associated Viral (AAV) vector type 9 delivery of Ex1-Q138-mutant huntingtin in the rat striatum as a short-time model for in vivo studies in drug discovery. Neurobiol Dis. 86:41–51. 2016.
Article4. Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 90:537–548. 1997.
Article5. Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 21:583–593. 2008.
Article6. de Almeida LP, Ross CA, Zala D, Aebischer P, Déglon N. Lentiviralmediated delivery of mutant huntingtin in the striatum of rats induces a selective neuropathology modulated by polyglutamine repeat size, huntingtin expression levels, and protein length. J Neurosci. 22:3473–3483. 2002.
Article7. DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 277:1990–1993. 1997.
Article8. Foster AC, Whetsell WO Jr, Bird ED, Schwarcz R. Quinolinic acid phosphoribosyltransferase in human and rat brain: activity in Huntington’s disease and in quinolinate-lesioned rat striatum. Brain Res. 336:207–214. 1985.
Article9. Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, et al. A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurode generation. Neuron. 23:181–192. 1999.
Article10. Hoth KF, Paulsen JS, Moser DJ, Tranel D, Clark LA, Bechara A. Patients with Huntington’s disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol. 29:365–376. 2007.
Article11. Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 33:2478–2484. 2002.
Article12. Jang M, Lee SE, Cho IH. Adeno-associated viral vector serotype DJmediated overexpression of N171-82Q-mutant huntingtin in the striatum of juvenile mice is a new model for Huntington’s disease. Front Cell Neurosci. 12:157. 2018.
Article13. Kim M. Brain Transplantation for Huntington Disease. Tissue Eng Regen Med. 2:322–326. 2005.14. MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 72:971–983. 1993.
Article15. Mangiarini L, Sathasivam K, Mahal A, Mott R, Seller M, Bates GP. Instability of highly expanded CAG repeats in mice transgenic for the Huntington’s disease mutation. Nat Genet. 15:197–200. 1997.
Article16. Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 87:493–506. 1996.
Article17. Marini B, Kertesz-Farkas A, Ali H, Lucic B, Lisek K, Manganaro L, et al. Nuclear architecture dictates HIV-1 integration site selection. Nature. 521:227–231. 2015.
Article18. Nussbaum RL, McInnes RR, Willard HF. Thompson & Thompson genetics in medicine. Philadelphia: Elsevier Health Sciences;2015.19. Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 15(5 Pt 2):3863–3875. 1995.
Article20. Palfi S, Brouillet E, Jarraya B, Bloch J, Jan C, Shin M, et al. Expression of mutated huntingtin fragment in the putamen is sufficient to produce abnormal movement in non-human primates. Mol Ther. 15:1444–1451. 2007.
Article21. Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Metaanalytical comparison of voxel-based morphometry studies in obsessivecompulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 67:701–711. 2010.
Article22. Ramaswamy S, McBride JL, Kordower JH. Animal models of Huntington’s disease. ILAR J. 48:356–373. 2007.
Article23. Reddy PH, Charles V, Williams M, Miller G, Whetsell WO Jr, Tagle DA. Transgenic mice expressing mutated full-length HD cDNA: a paradigm for locomotor changes and selective neuronal loss in Huntington’s disease. Philos Trans R Soc Lond B Biol Sci. 354:1035–1045. 1999.
Article24. Reddy PH, Williams M, Charles V, Garrett L, Pike-Buchanan L, Whetsell WO Jr, et al. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat Genet. 20:198–202. 1998.
Article25. Reddy PH, Williams M, Tagle DA. Recent advances in understanding the pathogenesis of Huntington’s disease. Trends Neurosci. 22:248–255. 1999.
Article26. Régulier E, Trottier Y, Perrin V, Aebischer P, Déglon N. Early and reversible neuropathology induced by tetracycline-regulated lentiviral overexpression of mutant huntingtin in rat striatum. Hum Mol Genet. 12:2827–2836. 2003.
Article27. Sanberg PR, Calderon SF, Giordano M, Tew JM, Norman AB. The quinolinic acid model of Huntington’s disease: locomotor abnormalities. Exp Neurol. 105:45–53. 1989.
Article28. Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 8:397–407. 1999.
Article29. Senut MC, Suhr ST, Kaspar B, Gage FH. Intraneuronal aggregate formation and cell death after viral expression of expanded polyglutamine tracts in the adult rat brain. J Neurosci. 20:219–229. 2000.
Article30. Shelbourne PF, Killeen N, Hevner RF, Johnston HM, Tecott L, Lewandoski M, et al. A Huntington’s disease CAG expansion at the murine Hdh locus is unstable and associated with behavioural abnormalities in mice. Hum Mol Genet. 8:763–774. 1999.
Article31. Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 12:1555–1567. 2003.
Article32. Stack EC, Ferrante RJ. Huntington’s disease: progress and potential in the field. Expert Opin Investig Drugs. 16:1933–1953. 2007.
Article33. Stack EC, Kubilus JK, Smith K, Cormier K, Signore SJD, Guelin E, et al. Chronology of behavioral symptoms and neuropathological sequela in R6/2 Huntington’s disease transgenic mice. J Comp Neurol. 490:354–370. 2005.
Article34. Vorisek I, Syka M, Vargova L. Brain diffusivity and structural changes in the R6/2 mouse model of huntington disease. J Neurosci Res. 95:1474–1484. 2017.
Article35. Wheeler VC, Auerbach W, White JK, Srinidhi J, Auerbach A, Ryan A, et al. Length-dependent gametic CAG repeat instability in the Huntington’s disease knock-in mouse. Hum Mol Genet. 8:115–122. 1999.
Article36. White JK, Auerbach W, Duyao MP, Vonsattel JP, Gusella JF, Joyner AL, et al. Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nat Genet. 17:404–410. 1997.
Article