J Korean Neurosurg Soc.
2020 Sep;63(5):566-578. 10.3340/jkns.2019.0187.
Dexamethasone Interferes with Autophagy and Affects Cell Survival in Irradiated Malignant Glioma Cells
- Affiliations
-
- 1Department of Cancer Control, 1 National Cancer Center Graduate School of Cancer Science and Policy, Goyang, Korea
- 2Department of Cancer Biomedical Science, 2 National Cancer Center Graduate School of Cancer Science and Policy, Goyang, Korea
- 3Neuro-oncology Clinic, 3 National Cancer Center, Goyang, Korea
- KMID: 2506020
- DOI: http://doi.org/10.3340/jkns.2019.0187
Abstract
Objective
: Radiation is known to induce autophagy in malignant glioma cells whether it is cytocidal or cytoprotective. Dexamethasone is frequently used to reduce tumor-associated brain edema, especially during radiation therapy. The purpose of the study was to determine whether and how dexamethasone affects autophagy in irradiated malignant glioma cells and to identify possible intervening molecular pathways.
Methods
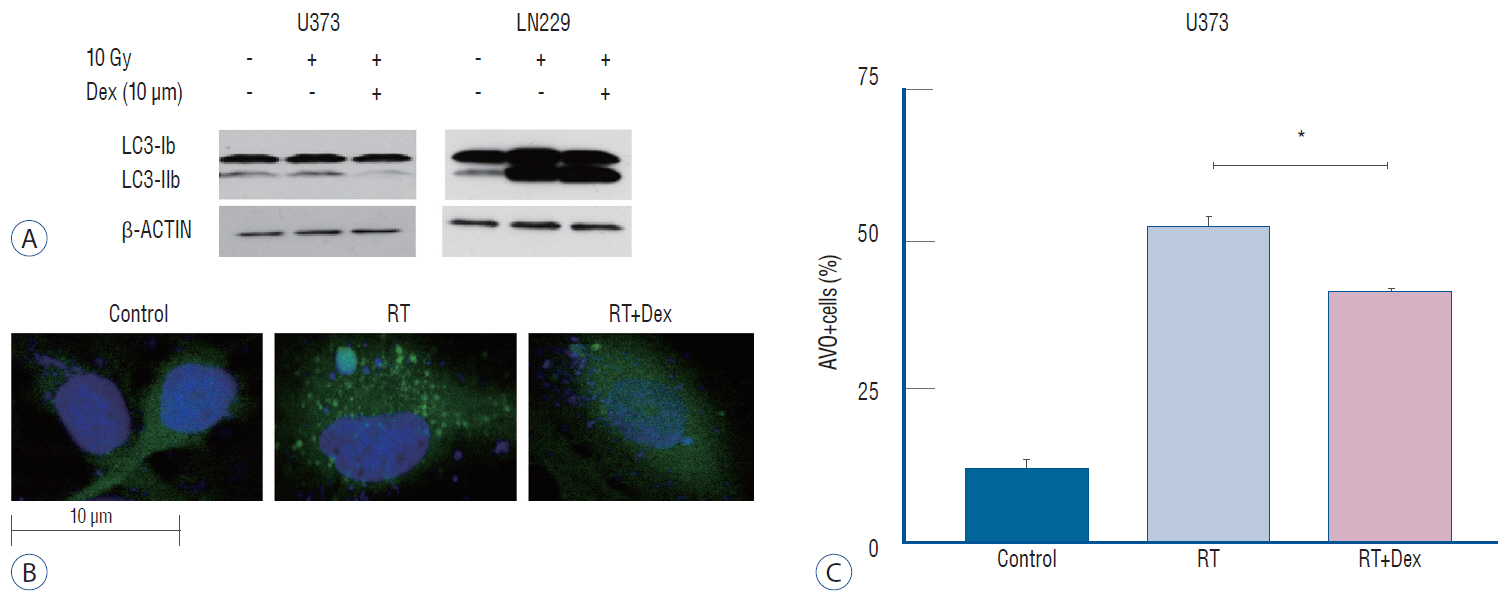
: We prepared p53 mutant U373 and LN229 glioma cell lines, which varied by phosphatase and tensin homolog (PTEN) mutational status and were used to make U373 stable transfected cells expressing GFP-LC3 protein. After performing cell survival assay after irradiation, the IC50 radiation dose was determined. Dexamethasone dose (10 µM) was determined from the literature and added to the glioma cells 24 hours before the irradiation. The effect of adding dexamethasone was evaluated by cell survival assay or clonogenic assay and cell cycle analysis. Measurement of autophagy was visualized by western blot of LC3-I/LC3-II and quantified by the GFP-LC3 punctuated pattern under fluorescence microscopy and acridine orange staining for acidic vesicle organelles by flow cytometry.
Results
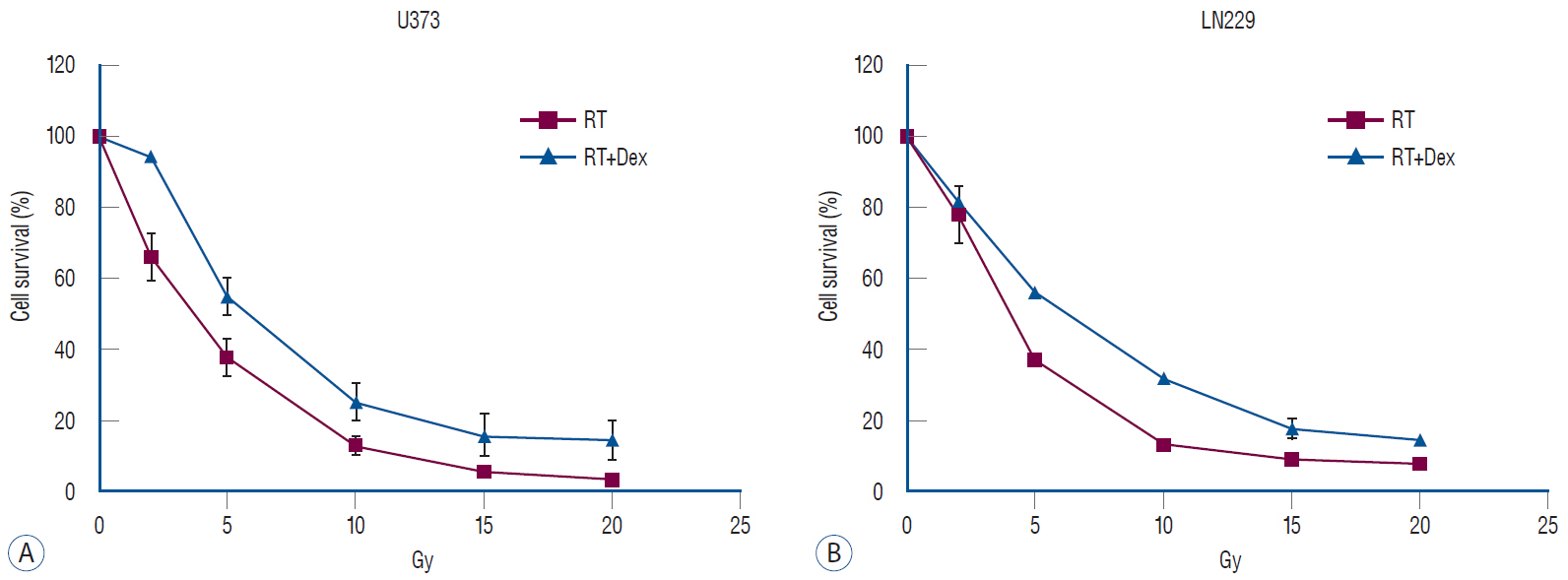
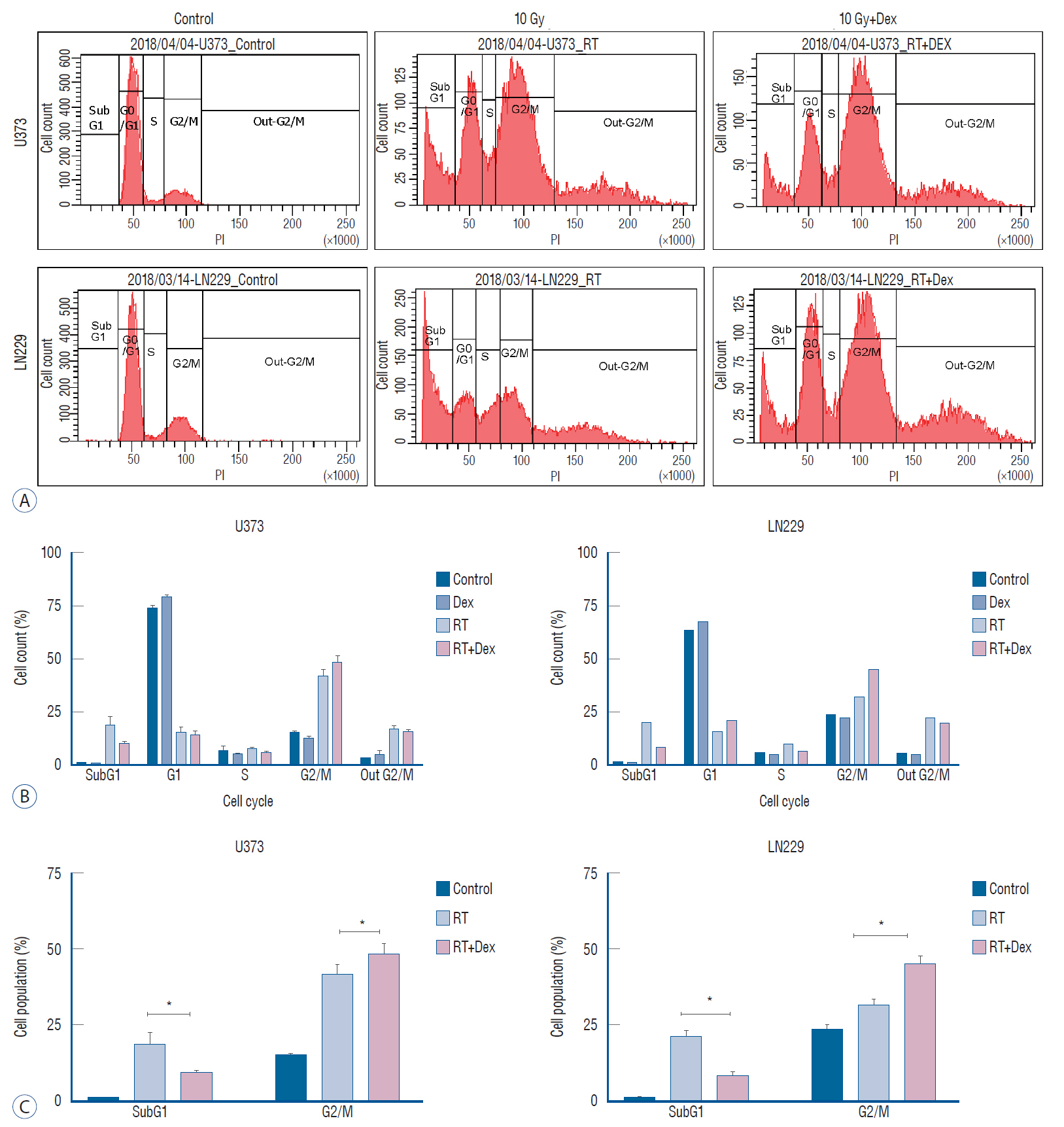
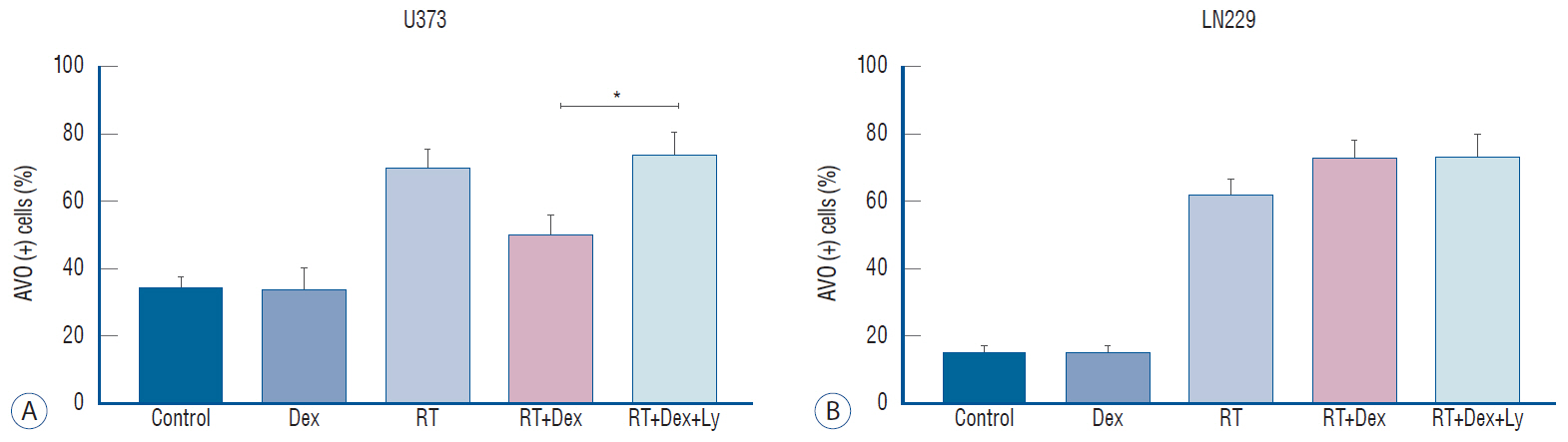
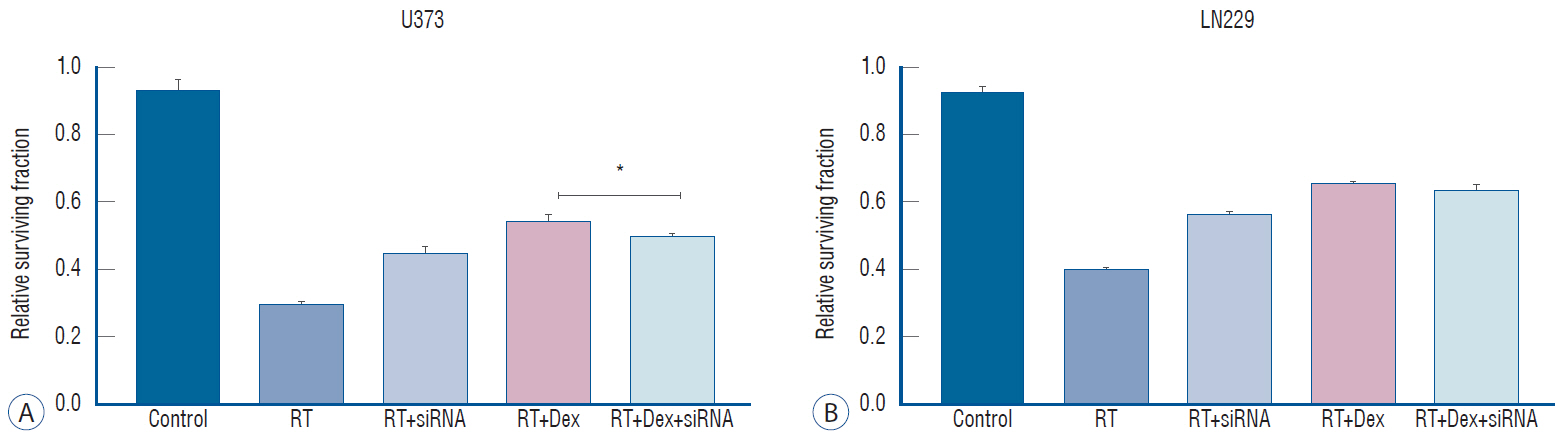
: Dexamethasone increased cell survival in both U373 and LN229 cells after irradiation. It interfered with autophagy after irradiation differently depending on the PTEN mutational status : the autophagy decreased in U373 (PTEN-mutated) cells but increased in LN229 (PTEN wild-type) cells. Inhibition of protein kinase B (AKT) phosphorylation after irradiation by LY294002 reversed the dexamethasone-induced decrease of autophagy and cell death in U373 cells but provoked no effect on both autophagy and cell survival in LN229 cells. After ATG5 knockdown, radiation-induced autophagy decreased and the effect of dexamethasone also diminished in both cell lines. The diminished autophagy resulted in a partial reversal of dexamethasone protection from cell death after irradiation in U373 cells; however, no significant change was observed in surviving fraction LN229 cells.
Conclusion
: Dexamethasone increased cell survival in p53 mutated malignant glioma cells and increased autophagy in PTENmutant malignant glioma cell but not in PTEN-wildtype cell. The difference of autophagy response could be mediated though the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin signaling pathway.
Keyword
Figure
Reference
-
References
1. Aasland D, Reich TR, Tomicic MT, Switzeny OJ, Kaina B, Christmann M. Repair gene O6 -methylguanine-DNA methyltransferase is controlled by SP1 and up-regulated by glucocorticoids, but not by temozolomide and radiation. J Neurochem. 144:139–151. 2018.
Article2. Alexanian R, Dimopoulos MA, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 80:887–890. 1992.
Article3. Andrade MV, Hiragun T, Beaven MA. Dexamethsaone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 172:7254–7262. 2004.
Article4. Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci. 1:24. 2014.
Article5. Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 9:297–308. 2008.
Article6. Cenciarini M, Valentino M, Belia S, Sforna L, Rosa P, Ronchetti S, et al. Dexamethasone in glioblastoma multiforme therapy: mechanisms and controversies. Front Mol Neurosci. 12:65. 2019.
Article7. Chen M, Nowak DG, Trotman LC. Molecular pathways: PI3K pathway phosphatases as biomarkers for cancer prognosis and therapy. Clin Cancer Res. 20:3057–3063. 2014.
Article8. Classen F, Kranz P, Riffkin H, Pompsch M, Wolf A, Göpelt K, et al. Autophagy induced by ionizing radiation promotes cell death over survival in human colorectal cancer cells. Exp Cell Res. 374:29–37. 2019.
Article9. Das A, Banik NL, Patel SJ, Ray SK. Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and preventing proteolytic activities. Mol Cancer. 3:36. 2004.10. Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 20(5 Suppl):S2–S8. 2016.
Article11. Errafiy R, Aguado C, Ghislat G, Esteve JM, Gil A, Loutfi M, et al. PTEN increases autophagy and inhibits the ubiquitin-proteasome pathway in glioma cells independently of its lipid phosphatase activity. PLoS One. 8:e83318. 2013.
Article12. Gewirtz DA. The autophagic response to radiation: relevance for radiation sensitization in cancer therapy. Radiat Res. 182:363–367. 2014.
Article13. Gustafsson AB, Gottlieb RA. Recycle or die: the role of autophagy in cardioprotection. J Mol Cell Cardiol. 44:654–661. 2008.
Article14. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 19:121–135. 2018.
Article15. Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 66:9349–9351. 2006.16. Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 11:1096–1106. 2010.
Article17. Jo GH, Bögler O, Chwae YJ, Yoo H, Lee SH, Park JB, et al. Radiation-induced autophagy contributes to cell death and induces apoptosis partly in malignant glioma cells. Cancer Res Treat. 47:221–241. 2015.
Article18. Kim KW, Mutter RW, Cao C, Albert JM, Freeman M, Hallahan DE, et al. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 281:36883–36890. 2006.
Article19. Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol. 4:43. 2009.
Article20. Luedi MM, Singh SK, Mosley JC, Hassan ISA, Hatami M, Gumin J, et al. Dexamethasone-mediated oncogenicity in vitro and in an animal model of glioblastoma. J Neurosurg. 129:1446–1455. 2018.
Article21. Mattern J, Büchler MW, Herr I. Cell cycle arrest by glucocorticoids may protect normal tissue and solid tumors from cancer therapy. Cancer Biol Ther. 6:1345–1354. 2007.
Article22. Milella M, Falcone I, Conciatori F, Cesta Incani U, Del Curatolo A, Inzerilli N, et al. PTEN: multiple functions in human malignant tumors. Front Oncol. 5:24. 2015.
Article23. Molitoris JK, McColl KS, Swerdlow S, Matsuyama M, Lam M, Finkel TH, et al. Glucocorticoid elevation of dexamethasone-induced gene 2 (Dig2/RTP801/REDD1) protein mediates autophagy in lymphocytes. J Biol Chem. 286:30181–30189. 2011.
Article24. Moretti L, Attia A, Kim KW, Lu B. Crosstalk between Bak/Bax and mTOR signaling regulates radiation-induced autophagy. Autophagy. 3:142–144. 2007.
Article25. Nakamura JL, Karlsson A, Arvold ND, Gottschalk AR, Pieper RO, Stokoe D, et al. PKB/Akt mediates radiosensitization by the signaling inhibitor LY294002 in human malignant gliomas. J Neurooncol. 71:215–222. 2005.
Article26. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 132:1033–1044. 2013.
Article27. Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 61:439–444. 2001.28. Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, et al. Corticosteroids compromise survival in glioblastoma. Brain. 139:1458–1471. 2016.
Article29. Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 90:1383–1435. 2010.
Article30. Shields LB, Shelton BJ, Shearer AJ, Chen L, Sun DA, Parsons S, et al. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol. 10:222. 2015.
Article31. Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 13:283–296. 2012.
Article32. Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol. 445:77–88. 2008.
Article33. Tóth GG, C Kloosterman C, Uges DR, Jonkman MF. Pharmacokinetics of high-dose oral and intravenous dexamethasone. Ther Drug Monit. 21:532–535. 1999.
Article34. Toulany M, Lee KJ, Fattah KR, Lin YF, Fehrenbacher B, Schaller M, et al. Akt promotes post-irradiation survival of human tumor cells through initiation, porgression, and termination of DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Res. 10:945–957. 2012.
Article35. Troncoso R, Paredes F, Parra V, Gatica D, Vasquez-Trincado C, Quiroga C, et al. Dexamethasone-induced autophagy mediates muscle atrophy through mitochondrial clearance. Cell Cycle. 13:2281–2295. 2014.
Article36. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 98:5116–5121. 2001.
Article37. Wang Z, Zhou L, Zheng X, Liu W. Effects of dexamethasone on autophagy and apoptosis in acute spinal cord injury. Neuroreport. 29:1084–1091. 2018.
Article38. Weller M. Glucocorticoid treatment of primary CNS lymphoma. J Neurooncol. 43:237–239. 1999.39. Willers H, Azzoli CG, Santivasi WL, Xia F. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J. 19:200–207. 2013.
Article40. Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, Beckman MJ, et al. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm Cancer. 2:272–285. 2011.
Article41. Yao KC, Komata T, Kondo Y, Kanzawa T, Kondo S, Germano IM. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. 98:378–384. 2003.
Article42. Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 8:1124–1132. 2006.
Article43. Zhang S, Liu Y, Liang Q. Low-dose dexamethasone affects osteoblast viability by inducing autophagy via intracellular ROS. Mol Med Rep. 17:4307–4316. 2018.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- in vitro Biological Response of Malignant Glioma Cell Lines to Gamma Knife Irradiation
- Radiation-Induced Autophagy Contributes to Cell Death and Induces Apoptosis Partly in Malignant Glioma Cells
- Memantine Induces NMDAR1-Mediated Autophagic Cell Death in Malignant Glioma Cells
- Curcumin-Induced Autophagy Augments Its Antitumor Effect against A172 Human Glioblastoma Cells
- Current Immunotherapeutic Approaches for Malignant Gliomas