Ann Surg Treat Res.
2020 Aug;99(2):118-126. 10.4174/astr.2020.99.2.118.
Nomogram for predicting overall survival in children with neuroblastoma based on SEER database
- Affiliations
-
- 1Department of Pediatric Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- KMID: 2504824
- DOI: http://doi.org/10.4174/astr.2020.99.2.118
Abstract
- Purpose
This study was performed to establish and validate a nomogram for predicting the overall survival in children with neuroblastoma.
Methods
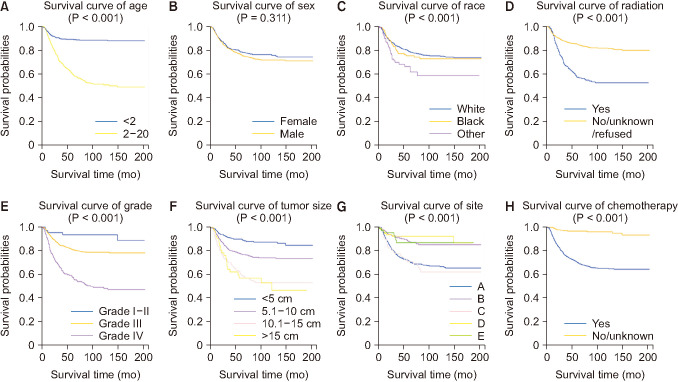
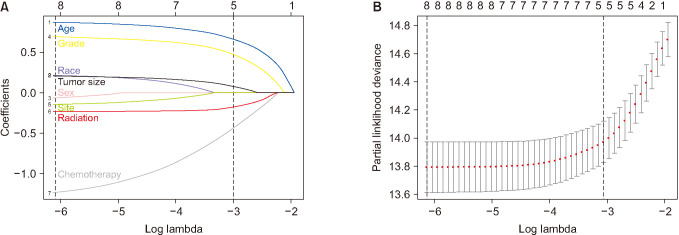
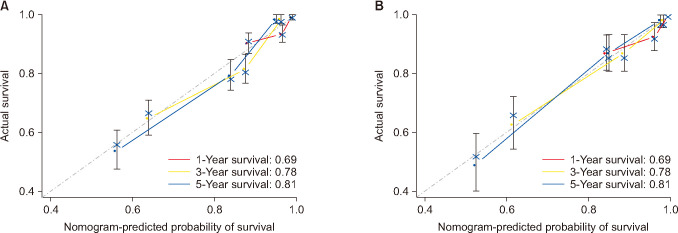
The latest clinical data of neuroblastoma in Surveillance, Epidemiology, and End Results (SEER) database was extracted from 2000 to 2016. The cases included were randomly divided into training and validation cohorts. The survival curves were drawn with a Kaplan-Meier estimator to investigate the influences of certain single factors on overall survival. Also, least absolute shrinkage and selection operator regression was applied to further select the prognostic variables for neuroblastoma. Additionally, receiver operating characteristic (ROC) curves and calibration curves were used to evaluate the accuracy of the nomogram.
Results
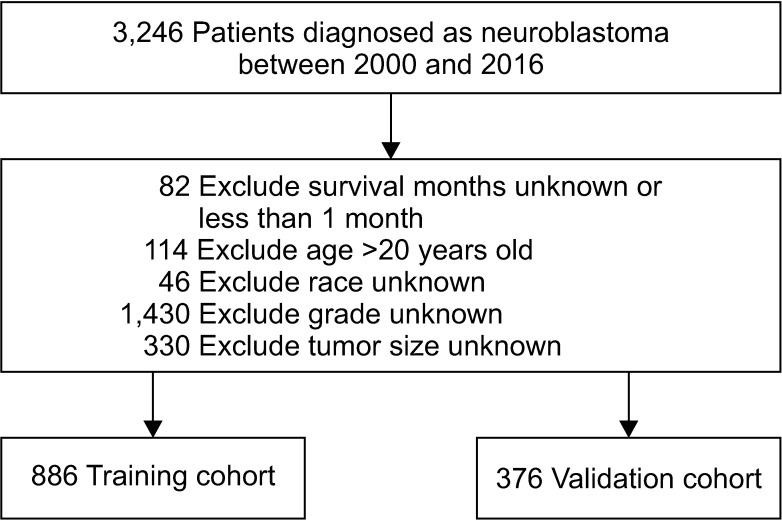
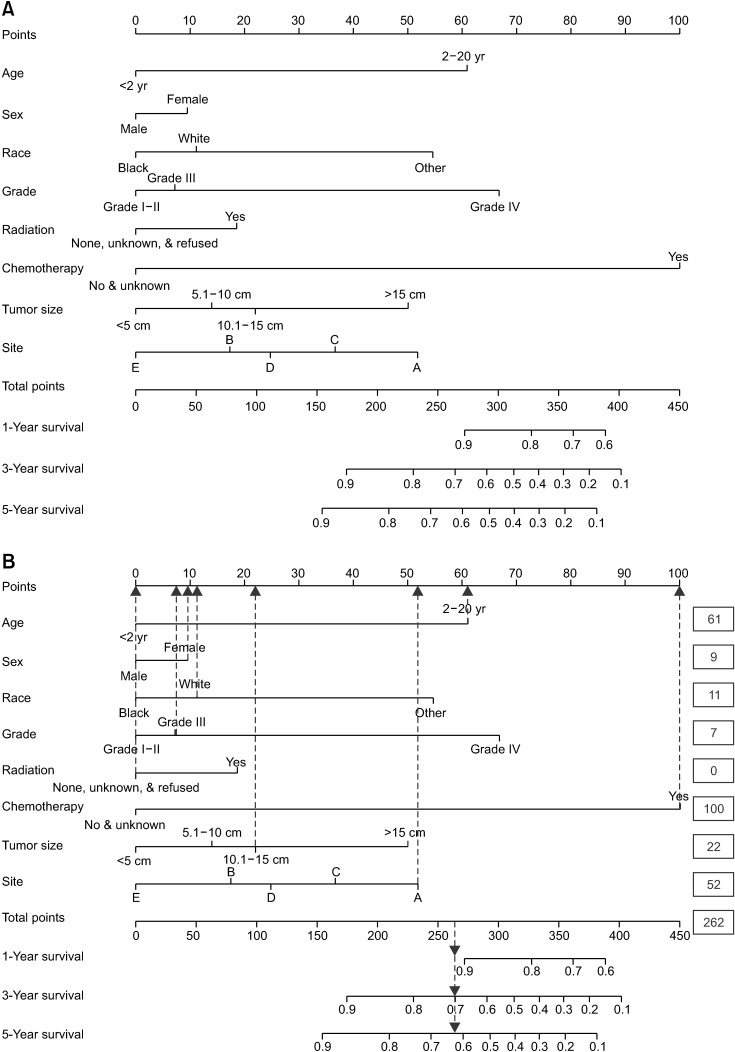
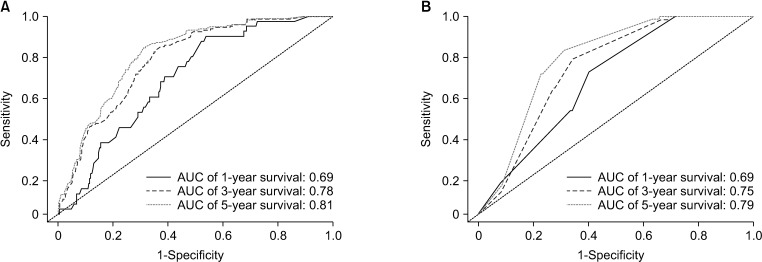
In total, 1,262 patients were collected and 8 independent prognostic factors were achieved, including patients’ age, sex, race, tumor grade, radiotherapy, chemotherapy, tumor site, and tumor size. Then we constructed a nomogram by using the data of the training cohort with 886 cases. Subsequently, the nomogram was validated internally and externally with 886 and 376 cases, respectively. The internal validation revealed that the area under the curves (AUC) of ROC curves of 1-, 3-, and 5-year overall survival were 0.69, 0.78, and 0.81, respectively. Accordingly, the external validation also showed that the AUC of 1-, 3-, and 5-year overall survival were all ≥0.69. Both methods of validation demonstrated that the predictive calibration curves were consistent with standard curves.
Conclusion
The nomogram possess the potential to be a new tool in predicting the survival rate of neuroblastoma patients.
Keyword
Figure
Reference
-
1. Jubierre L, Jiménez C, Rovira E, Soriano A, Sábado C, Gros L, et al. Targeting of epigenetic regulators in neuroblastoma. Exp Mol Med. 2018; 50:51. PMID: 29700278.2. Rickman DS, Schulte JH, Eilers M. The expanding world of N-MYC-driven tumors. Cancer Discov. 2018; 8:150–163. PMID: 29358508.3. Meena JP, Gupta AK. Neuroblastoma in a developing country: miles to go. Indian J Pediatr. 2019; 86:403–405. PMID: 30915646.4. Zareifar S, Shakibazad N, Zekavat OR, Bordbar M, Shahriari M. Successful treatment of refractory metastatic neuroblastoma with panobinostat in combination with chemotherapy agents and iodine-131-meta-iodobenzylguanidine therapy. J Oncol Pharm Pract. 2020; 26:481–486. PMID: 31156056.5. Herd F, Basta NO, McNally RJQ, Tweddle DA. A systematic review of re-induction chemotherapy for children with relapsed high-risk neuroblastoma. Eur J Cancer. 2019; 111:50–58. PMID: 30822684.6. Swift CC, Eklund MJ, Kraveka JM, Alazraki AL. Updates in diagnosis, management, and treatment of neuroblastoma. Radiographics. 2018; 38:566–580. PMID: 29528815.7. PDQ Pediatric Treatment Editorial Board. Neuroblastoma Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US);2002.8. Valter K, Zhivotovsky B, Gogvadze V. Cell death-based treatment of neuroblastoma. Cell Death Dis. 2018; 9:113. PMID: 29371588.9. Pastor ER, Mousa SA. Current management of neuroblastoma and future direction. Crit Rev Oncol Hematol. 2019; 138:38–43. PMID: 31092383.10. Han Y, Ye X, Cheng J, Zhang S, Feng W, Han Z, et al. Integrative analysis based on survival associated co-expression gene modules for predicting Neuroblastoma patients' survival time. Biol Direct. 2019; 14:4. PMID: 30760313.11. Yao W, Li K, Dong KR, Zheng S, Xiao XM. Long-term prognosis of low-risk neuroblastoma treated by surgery alone: an experience from a single institution of China. World J Pediatr. 2019; 15:148–152. PMID: 30446974.12. Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015; 33:3008–3017. PMID: 26304901.13. Morgenstern DA, Bagatell R, Cohn SL, Hogarty MD, Maris JM, Moreno L, et al. The challenge of defining “ultra-high-risk” neuroblastoma. Pediatr Blood Cancer. 2019; 66:e27556. PMID: 30479064.14. Park JR, Bagatell R, Cohn SL, Pearson AD, Villablanca JG, Berthold F, et al. Revisions to the international neuroblastoma response criteria: a consensus statement from the National Cancer Institute clinical trials planning meeting. J Clin Oncol. 2017; 35:2580–2587. PMID: 28471719.15. Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. Neuroblastoma. Nat Rev Dis Primers. 2016; 2:16078. PMID: 27830764.16. Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009; 27:289–297. PMID: 19047291.17. Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993; 11:1466–1477. PMID: 8336186.18. Hu CY, Pan ZY, Yang J, Chu XH, Zhang J, Tao XJ, et al. Nomograms for predicting long-term overall survival and cancer-specific survival in lip squamous cell carcinoma: a population-based study. Cancer Med. 2019; 8:4032–4042. PMID: 31112373.19. Liu G, Liu Q, Sun SR. Nomograms for estimating survival in patients with papillary thyroid cancer after surgery. Cancer Manag Res. 2019; 11:3535–3544. PMID: 31114382.20. Huang L, Balavarca Y, van der Geest L, Lemmens V, Van Eycken L, De Schutter H, et al. Development and validation of a prognostic model to predict the prognosis of patients who underwent chemotherapy and resection of pancreatic adenocarcinoma: a large international population-based cohort study. BMC Med. 2019; 17:66. PMID: 30905320.21. Guo CX, Shen YN, Zhang Q, Zhang XZ, Wang JL, Gao SL, et al. Prediction of postoperative pancreatic fistula using a nomogram based on the updated definition. Ann Surg Treat Res. 2020; 98:72–81. PMID: 32051815.22. Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019; 17:479–505. PMID: 31085757.23. Xie Y, Xu H, Fang F, Li Z, Zhou H, Pan J, et al. A 3-protein expression signature of neuroblastoma for outcome prediction. Am J Surg Pathol. 2018; 42:1027–1035. PMID: 29794872.24. Li X, Meng Y. A prognostic nomogram for neuroblastoma in children. PeerJ. 2019; 7:e7316. PMID: 31338261.25. Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997; 16:385–395. PMID: 9044528.26. Hu J, Wang T, Zhang KH, Jiang YP, Xu S, Chen SH, et al. Pretreatment risk management of a novel nomogram model for prediction of thoracoabdominal extrahepatic metastasis in primary hepatic carcinoma. J Transl Med. 2019; 17:117. PMID: 30961629.27. Jiang Y, Wang W, Chen C, Zhang X, Zha X, Lv W, et al. Radiomics signature on computed tomography imaging: association with lymph node metastasis in patients with gastric cancer. Front Oncol. 2019; 9:340. PMID: 31106158.28. London WB, Castel V, Monclair T, Ambros PF, Pearson AD, Cohn SL, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011; 29:3286–3292. PMID: 21768459.29. Louis CU, Shohet JM. Neuroblastoma: molecular pathogenesis and therapy. Annu Rev Med. 2015; 66:49–63. PMID: 25386934.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nomogram for Predicting Survival for Oral Squamous Cell Carcinoma
- Epidemiologic characteristics and a prognostic nomogram for patients with vulvar cancer: results from the Surveillance, Epidemiology, and End Results (SEER) program in the United States, 1975 to 2016
- A personalized prognostic model for long-term survival in patients with intrahepatic cholangiocarcinoma: a retrospective cohort study
- A Nomogram for Predicting Overall Survival of Patients With Primary Spinal Cord Glioblastoma
- A personalized nomogram for predicting 3-year overall survival of patients with uterine carcinosarcoma in a tertiary care hospital in Southern Thailand