Pathogenesis and clinical perspectives of extraintestinal manifestations in inflammatory bowel diseases
- Affiliations
-
- 1Department of Internal Medicine and Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
- 2Avison Biomedical Research Center, Severance Hospital, Seoul, Korea
- 3Affiliate Faculty, Pohang University of Science and Technology (POSTECH), Pohang, Korea
- KMID: 2504580
- DOI: http://doi.org/10.5217/ir.2019.00128
Abstract
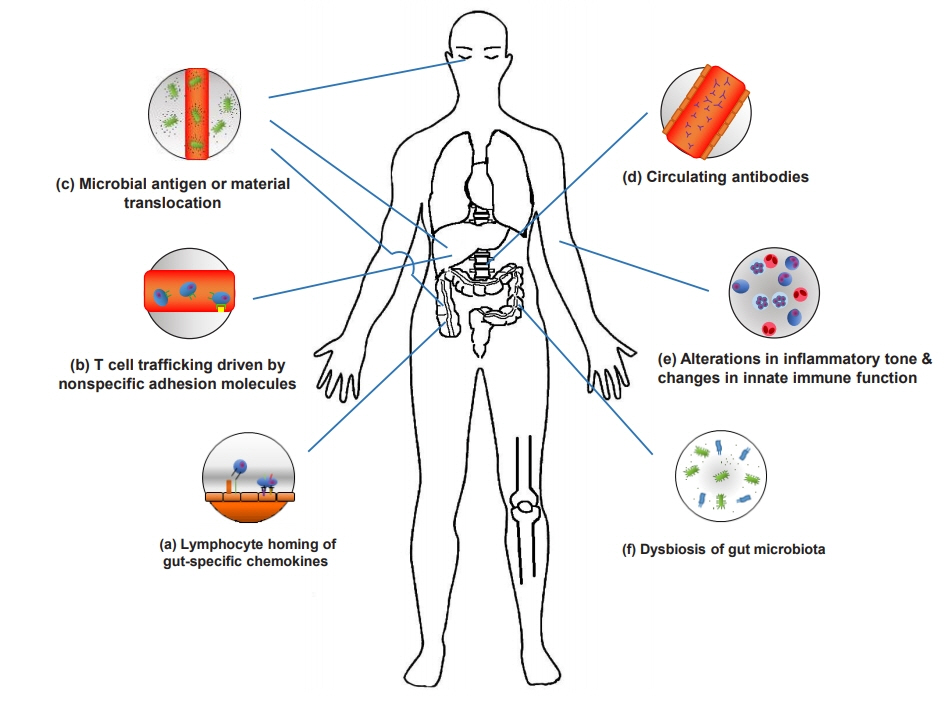
- A considerable number of patients with inflammatory bowel disease (IBD) experience extraintestinal manifestations (EIMs), which can present either before or after IBD diagnosis. Unraveling the pathogenic pathways of EIMs in IBD is challenging because of the lack of reliable criteria for diagnosis and difficulty in distinguishing EIMs from external pathologies caused by drugs or other etiologies. Optimizing treatment can also be difficult. Early diagnosis and management of EIM revolve around multidisciplinary teams, and they should have the resources necessary to make and implement appropriate decisions. In addition, specialists of the affected organs should be trained in IBD treatment. Furthermore, patient awareness regarding the extraintestinal symptoms of IBD is of paramount importance for improving patient understanding of disease and health outcomes. Herein, we review the pathogenesis and clinical perspectives of EIMs in IBD.
Keyword
Figure
Cited by 8 articles
-
KASID Guidance for Clinical Practice Management of Adult Inflammatory Bowel Disease during the COVID-19 Pandemic: Expert Consensus Statement
Yong Eun Park, Yoo Jin Lee, Ji Young Chang, Hyun Joo Song, Duk Hwan Kim, Young Joo Yang, Byung Chang Kim, Jae Gon Lee, Hee Chan Yang, Miyoung Choi, Seong-Eun Kim, Seung-Jae Myung, The Clinical Practice Guideline Committee of the K Diseases
Korean J Gastroenterol. 2021;78(2):105-116. doi: 10.4166/kjg.2021.112.Functional Gastrointestinal Disorders in Patients with Inflammatory Bowel Disease
Kyeong Ok Kim
Korean J Gastroenterol. 2022;79(1):4-11. doi: 10.4166/kjg.2022.001.Is primary sclerosing cholangitis with inflammatory bowel disease different between patients in the East and West?
Yong Eun Park
Intest Res. 2022;20(2):157-158. doi: 10.5217/ir.2022.00041.Korean Association for the Study of Intestinal Diseases guidance for clinical practice of adult inflammatory bowel disease during the coronavirus disease 2019 pandemic: expert consensus statements
Yong Eun Park, Yoo Jin Lee, Ji Young Chang, Hyun Joo Song, Duk Hwan Kim, Young Joo Yang, Byung Chang Kim, Jae Gon Lee, Hee Chan Yang, Miyoung Choi, Seong-Eun Kim, Seung-Jae Myung
Intest Res. 2022;20(4):431-444. doi: 10.5217/ir.2021.00111.Risks of colorectal cancer and biliary cancer according to accompanied primary sclerosing cholangitis in Korean patients with ulcerative colitis: a nationwide population-based study
Eun Hye Oh, Ye-Jee Kim, Minju Kim, Seung Ha Park, Tae Oh Kim, Sang Hyoung Park
Intest Res. 2023;21(2):252-265. doi: 10.5217/ir.2022.00092.Are the risks of colorectal cancer and biliary cancer really increased if patients with ulcerative colitis have primary sclerosing cholangitis?
Jung Wook Lee, Won Moon
Intest Res. 2023;21(2):171-173. doi: 10.5217/ir.2023.00049.Treatment of primary sclerosing cholangitis combined with inflammatory bowel disease
You Sun Kim, Edward H. Hurley, Yoojeong Park, Sungjin Ko
Intest Res. 2023;21(4):420-432. doi: 10.5217/ir.2023.00039.Regional variations in the prevalence of primary sclerosing cholangitis associated with inflammatory bowel disease
Kwang Woo Kim, Hyoun Woo Kang
Intest Res. 2023;21(4):413-414. doi: 10.5217/ir.2023.00133.
Reference
-
1. Hedin CRH, Vavricka SR, Stagg AJ, et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohns Colitis. 2019; 13:541–554.
Article2. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:1982–1992.
Article3. Yang BR, Choi NK, Kim MS, et al. Prevalence of extraintestinal manifestations in Korean inflammatory bowel disease patients. PLoS One. 2018; 13:e0200363.
Article4. Severs M, Spekhorst LM, Mangen MJ, et al. Sex-related differences in patients with inflammatory bowel disease: results of 2 prospective cohort studies. Inflamm Bowel Dis. 2018; 24:1298–1306.
Article5. Vavricka SR, Rogler G, Gantenbein C, et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the swiss inflammatory bowel disease cohort. Inflamm Bowel Dis. 2015; 21:1794–1800.
Article6. Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Crohn’s disease in the elderly: a comparison with young adults. J Clin Gastroenterol. 1998; 27:129–133.7. Zeitz J, Ak M, Müller-Mottet S, et al. Pain in IBD patients: very frequent and frequently insufficiently taken into account. PLoS One. 2016; 11:e0156666.
Article8. Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015; 67:128–139.
Article9. Tito RY, Cypers H, Joossens M, et al. Brief report: dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol. 2017; 69:114–121.
Article10. Viladomiu M, Kivolowitz C, Abdulhamid A, et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci Transl Med. 2017; 9:e. –aaf9655.
Article11. Ciccia F, Guggino G, Rizzo A, et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis. 2015; 74:1739–1747.
Article12. Cuthbert RJ, Fragkakis EM, Dunsmuir R, et al. Brief report: group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol. 2017; 69:1816–1822.
Article13. Kummen M, Holm K, Anmarkrud JA, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017; 66:611–619.
Article14. Nakamoto N, Sasaki N, Aoki R, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019; 4:492–503.
Article15. Dodd D, Spitzer MH, Van Treuren W, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017; 551:648–652.
Article16. King SJ, McCole DF. Epithelial-microbial diplomacy: escalating border tensions drive inflammation in inflammatory bowel disease. Intest Res. 2019; 17:177–191.
Article17. Godefroy E, Alameddine J, Montassier E, et al. Expression of CCR6 and CXCR6 by gut-derived CD4+/CD8α+ T-regulatory cells, which are decreased in blood samples from patients with inflammatory bowel diseases. Gastroenterology. 2018; 155:1205–1217.
Article18. Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006; 6:244–251.
Article19. Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993; 74:185–195.
Article20. Svensson M, Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002; 110:1113–1121.
Article21. Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006; 27:235–243.
Article22. Chapman RW. Aetiology and natural history of primary sclerosing cholangitis: a decade of progress? Gut. 1991; 32:14331435.23. Chen Q, Fisher DT, Clancy KA, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006; 7:1299–1308.
Article24. Henninger DD, Panés J, Eppihimer M, et al. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997; 158:1825–1832.
Article25. Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond). 1998; 94:461–471.
Article26. Nikolaus S, Bauditz J, Gionchetti P, Witt C, Lochs H, Schreiber S. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut. 1998; 42:470–476.
Article27. Smith AM, Rahman FZ, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009; 206:1883–1897.
Article28. Sanders TJ, McCarthy NE, Giles EM, et al. Increased production of retinoic acid by intestinal macrophages contributes to their inflammatory phenotype in patients with Crohn’s disease. Gastroenterology. 2014; 146:1278–1288.
Article29. Espaillat MP, Kew RR, Obeid LM. Sphingolipids in neutrophil function and inflammatory responses: mechanisms and implications for intestinal immunity and inflammation in ulcerative colitis. Adv Biol Regul. 2017; 63:140–155.
Article30. Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017; 17:248–261.
Article31. Ciccia F, Accardo-Palumbo A, Alessandro R, et al. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 2012; 64:1869–1878.
Article32. Kenna TJ, Davidson SI, Duan R, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012; 64:1420–1429.
Article33. Salmi M, Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J Immunol. 2001; 166:4650–4657.
Article34. Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002; 169:983–992.
Article35. Soriano A, Salas A, Salas A, et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000; 80:1541–1551.
Article36. Jacques P, McGonagle D. The role of mechanical stress in the pathogenesis of spondyloarthritis and how to combat it. Best Pract Res Clin Rheumatol. 2014; 28:703–710.
Article37. Scofield RH, Kurien B, Gross T, Warren WL, Harley JB. HLAB27 binding of peptide from its own sequence and similar peptides from bacteria: implications for spondyloarthropathies. Lancet. 1995; 345:1542–1544.
Article38. Ramos M, Alvarez I, Sesma L, Logean A, Rognan D, López de Castro JA. Molecular mimicry of an HLA-B27-derived ligand of arthritis-linked subtypes with chlamydial proteins. J Biol Chem. 2002; 277:37573–37581.
Article39. Oshitani N, Watanabe K, Nakamura S, Higuchi K, Arakawa T. Extraintestinal complications in patients with ulcerative colitis. Nihon Rinsho. 2005; 63:874–878.40. Bhagat S, Das KM. A shared and unique peptide in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology. 1994; 107:103–108.
Article41. Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, et al. Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity. 2015; 43:343–353.
Article42. Nakamura YK, Janowitz C, Metea C, et al. Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Sci Rep. 2017; 7:11745.
Article43. Reeves E, James E. The role of polymorphic ERAP1 in autoinflammatory disease. Biosci Rep. 2018; 38–BSR20171503.44. Martin TM, Smith JR, Rosenbaum JT. Anterior uveitis: current concepts of pathogenesis and interactions with the spondyloarthropathies. Curr Opin Rheumatol. 2002; 14:337–337341.
Article45. Peeters H, Vander Cruyssen B, Laukens D, et al. Radiological sacroiliitis, a hallmark of spondylitis, is linked with CARD15 gene polymorphisms in patients with Crohn’s disease. Ann Rheum Dis. 2004; 63:1131–1134.
Article46. Moon CM, Cheon JH, Kim SW, et al. Association of signal transducer and activator of transcription 4 genetic variants with extra-intestinal manifestations in inflammatory bowel disease. Life Sci. 2010; 86:661–667.
Article47. De Vos M, De Keyser F, Mielants H, Cuvelier C, Veys E. Review article: bone and joint diseases in inflammatory bowel disease. Aliment Pharmacol Ther. 1998; 12:397–404.48. Palm O, Moum B, Ongre A, Gran JT. Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: a population study (the IBSEN study). J Rheumatol. 2002; 29:511–515.49. van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009; 68:18111–1818.
Article50. Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006; 101:1012–1023.
Article51. Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore). 1976; 55:401–412.
Article52. Trost LB, McDonnell JK. Important cutaneous manifestations of inflammatory bowel disease. Postgrad Med J. 2005; 81:580–585.
Article53. Hopkins DJ, Horan E, Burton IL-, Clamp SE, de Dombal FT, Goligher JC. Ocular disorders in a series of 332 patients with Crohn’s disease. Br J Ophthalmol. 1974; 58:732–737.
Article54. Kupferschmidt H, Langenegger T, Krähenbühl S. Pericarditis in chronic inflammatory bowel disease: underlying disease or side effects of therapy? Clinical problem solving. Schweiz Med Wochenschr. 1996; 126:2184–2190.55. Brown G. 5-Aminosalicylic acid-associated myocarditis and pericarditis: a narrative review. Can J Hosp Pharm. 2016; 69:466–472.
Article56. Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol. 2012; 59:779–792.
Article57. Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007; 50:19141931.
Article58. Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis. 2014; 8:469–479.
Article59. Singh S, Singh H, Loftus EV Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and metaanalysis. Clin Gastroenterol Hepatol. 2014; 12:382–393.
Article60. Oussalah A, Guéant JL, Peyrin-Biroulet L. Meta-analysis: hyperhomocysteinaemia in inflammatory bowel diseases. Aliment Pharmacol Ther. 2011; 34:1173–1184.
Article61. Tan VP, Chung A, Yan BP, Gibson PR. Venous and arterial disease in inflammatory bowel disease. J Gastroenterol Hepatol. 2013; 28:1095–1113.
Article62. Mendes FD, Levy C, Enders FB, Loftus EV Jr, Angulo P, Lindor KD. Abnormal hepatic biochemistries in patients with inflammatory bowel disease. Am J Gastroenterol. 2007; 102:344–350.
Article63. Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991; 11:31–39.
Article64. Loftus EV Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005; 54:91–96.
Article65. Park YE, Cheon JH, Park JJ, et al. Risk factors and clinical courses of concomitant primary sclerosing cholangitis and ulcerative colitis: a Korean multicenter study. Int J Colorectal Dis. 2018; 33:1497–1500.
Article66. Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016; 10:239254.
Article67. Woodward J, Neuberger J. Autoimmune overlap syndromes. Hepatology. 2001; 33:994–1002.
Article68. Gregorio GV, Portmann B, Karani J, et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001; 33:544–553.
Article69. Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009; 15:63–68.
Article70. Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010; 51:21932213.
Article71. Braun M, Fraser GM, Kunin M, Salamon F, Tur-Kaspa R. Mesalamine-induced granulomatous hepatitis. Am J Gastroenterol. 1999; 94:1973–1974.
Article72. Ramos LR, Sachar DB, DiMaio CJ, Colombel JF, Torres J. Inflammatory bowel disease and pancreatitis: a review. J Crohns Colitis. 2016; 10:95–104.
Article73. Klein A, Eliakim R. Non steroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals (Basel). 2010; 3:1084–1092.
Article74. El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006; 101:311–317.
Article75. Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev. 2005; (2):CD004800.
Article76. Dougados M, vam der Linden S, Leirisalo-Repo M, et al. Sulfasalazine in the treatment of spondyloarthropathy: a randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995; 38:618–627.77. Baraliakos X, Braun J. Biologic therapies for spondyloarthritis: what is new? Curr Rheumatol Rep. 2012; 14:422–427.
Article78. Kaufman I, Caspi D, Yeshurun D, Dotan I, Yaron M, Elkayam O. The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatol Int. 2005; 25:406–410.
Article79. Baumgart DC, Wiedenmann B, Dignass AU. Rescue therapy with tacrolimus is effective in patients with severe and refractory inflammatory bowel disease. Aliment Pharmacol Ther. 2003; 17:1273–1281.
Article80. Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. BMJ. 2006; 333:181–184.
Article81. Mintz R, Feller ER, Bahr RL, Shah SA. Ocular manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2004; 10:135–139.
Article82. Fries W, Giofré MR, Catanoso M, Lo Gullo R. Treatment of acute uveitis associated with Crohn’s disease and sacroileitis with infliximab. Am J Gastroenterol. 2002; 97:499–500.
Article83. Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014; 146:835–848.
Article84. Chandok N, Hirschfield GM. Management of primary sclerosing cholangitis: conventions and controversies. Can J Gastroenterol. 2012; 26:261–268.
Article85. Brand M, Bizos D, O’Farrell P Jr. Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database Syst Rev. 2010; (10):CD007345.
Article86. Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a metaanalysis. Gastrointest Endosc. 2002; 56:48–54.
Article87. Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013; 7:1–33.
Article88. Peyrin-Biroulet L, Van Assche G, Gómez-Ulloa D, et al. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017; 15:25–36.
Article89. de Gannes GC, Ghoreishi M, Pope J, et al. Psoriasis and pustular dermatitis triggered by TNF-{alpha} inhibitors in patients with rheumatologic conditions. Arch Dermatol. 2007; 143:223–231.90. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018; 9:25.
Article91. Greuter T, Navarini A, Vavricka SR. Skin manifestations of inflammatory bowel disease. Clin Rev Allergy Immunol. 2017; 53:413–427.
Article92. Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009; 330:864–875.
Article93. Sody E, Körber A. Psoriasis induced by vedolizumab. Inflamm Bowel Dis. 2017; 23–E9-E11.
Article94. Vavricka SR, Galván JA, Dawson H, et al. Expression patterns of TNFα, MAdCAM1, and STAT3 in intestinal and skin manifestations of inflammatory bowel disease. J Crohns Colitis. 2018; 12:347–354.
Article95. Chateau T, Bonovas S, Le Berre C, Mathieu N, Danese S, Peyrin-Biroulet L. Vedolizumab treatment in extra-intestinal manifestations in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2019; 13:1569–1577.
Article96. Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebocontrolled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis. 2016; 75:1984–1988.
Article97. Greb JE, Gottlieb AB, Goldminz AM. High-dose ustekinumab for the treatment of severe, recalcitrant pyoderma gangrenosum. Dermatol Ther. 2016; 29:482–483.
Article98. Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibodyinduced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γexpressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014; 63:567–577.
Article99. Čarija A, Ivić I, Marasović-Krstulović D, Puizina-Ivić N. Paradoxical psoriatic arthritis in a patient with psoriasis treated with ustekinumab. Rheumatology (Oxford). 2015; 54:21142116.100. Gregoriou S, Kazakos C, Christofidou E, Kontochristopoulos G, Vakis G, Rigopoulos D. Pustular psoriasis development after initial ustekinumab administration in chronic plaque psoriasis. Eur J Dermatol. 2011; 21:104–105.
Article101. Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn’s disease: results of the ICC registry, a nationwide prospective observational cohort study. J Crohns Colitis. 2020; 14:33–45.
Article102. Peyrin-Biroulet L, Danese S, Louis E, et al. DOP50 Effect of upadacitinib on extra-intestinal manifestations in patients with moderate to severe Crohn’s disease: data from the CELEST study. J Crohns Colitis. 2019; 13–Suppl 1:S057.103. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, headto-head, randomised controlled trial. Lancet. 2017; 390:457468.104. Satta R, Pes GM, Rocchi C, Pes MC, Dore MP. Is probiotic use beneficial for skin lesions in patients with inflammatory bowel disease? J Dermatolog Treat. 2019; 30:612–616.
Article105. Sanges M, Valente G, Rea M, et al. Probiotics in spondyloarthropathy associated with ulcerative colitis: a pilot study. Eur Rev Med Pharmacol Sci. 2009; 13:233–234.106. Manser CN, Borovicka J, Seibold F, et al. Risk factors for complications in patients with ulcerative colitis. United European Gastroenterol J. 2016; 4:281–287.
Article107. Huang V, Mishra R, Thanabalan R, Nguyen GC. Patient awareness of extraintestinal manifestations of inflammatory bowel disease. J Crohns Colitis. 2013; 7:e318–e324.
Article108. Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011; 106:713–718.
Article109. Kelso M, Feagins LA. Can smartphones help deliver smarter care for patients with inflammatory bowel disease? Inflamm Bowel Dis. 2018; 24:1453–1459.
Article110. Walsh A, Matini L, Hinds C, et al. Real-time data monitoring for ulcerative colitis: patient perception and qualitative analysis. Intest Res. 2019; 17:365–374.
Article111. Atreja A, Otobo E, Ramireddy K, Deorocki A. Remote patient monitoring in IBD: current state and future directions. Curr Gastroenterol Rep. 2018; 20:6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dermatologic Manifestations in Inflammatory Bowel Disease
- Quality of life in inflammatory bowel diseases: it is not all about the bowel
- Comprehensive Association Analysis of Extraintestinal Manifestations in Inflammatory Bowel Disease: Recent Findings and Clinical Challenges
- Pathogenesis of Inflammatory Bowel Diseases
- Extraintestinal Cutaneous Manifestations in Inflammatory Bowel Disease: Survey and Literature Review