Korean J Physiol Pharmacol.
2020 Jul;24(4):363-372. 10.4196/kjpp.2020.24.4.363.
Gardenia jasminoides extract and its constituent, genipin, inhibit activation of CD3/CD28 co-stimulated CD4+ T cells via ORAI1 channel
- Affiliations
-
- 1Channelopathy Research Center (CRC), Dongguk University College of Medicine, Korea
- 2Department of Internal Medicine, Graduate School of Medicine, Dongguk University, Goyang 10326, Korea
- 3Department of Physiology, Dongguk University College of Medicine, Gyeongju 38066, Korea
- KMID: 2503329
- DOI: http://doi.org/10.4196/kjpp.2020.24.4.363
Abstract
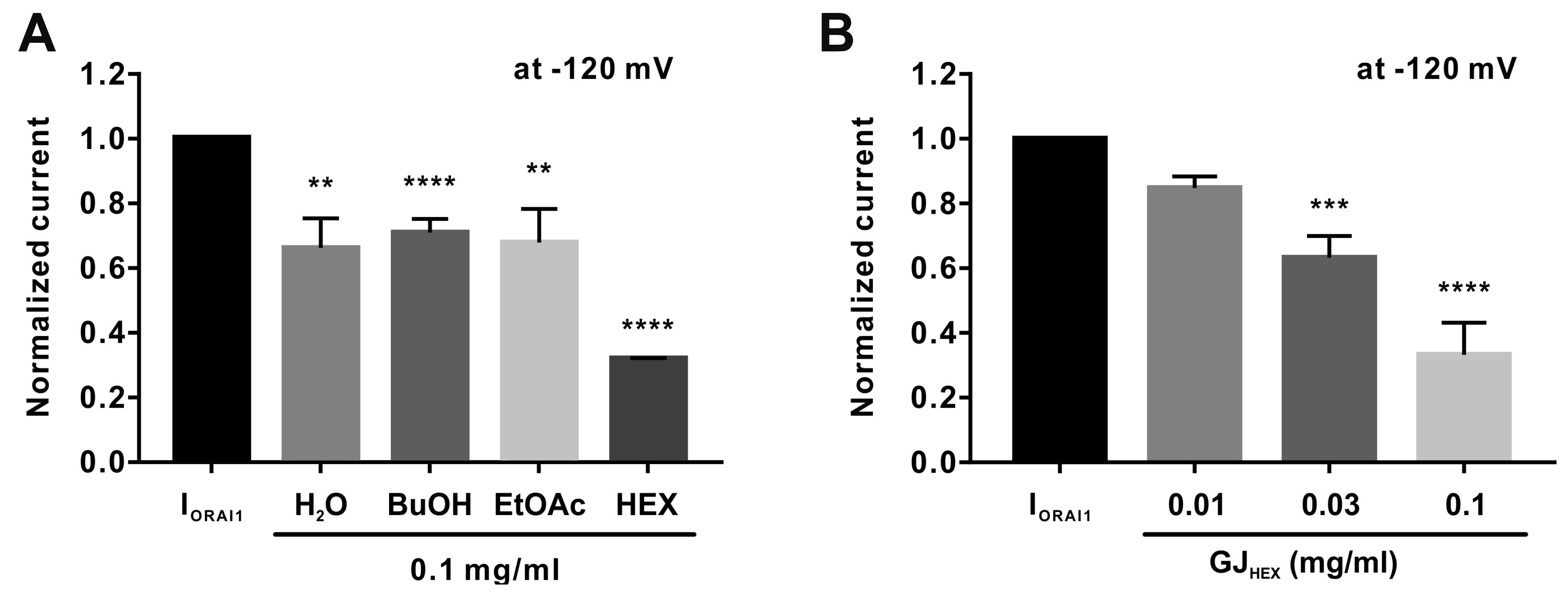
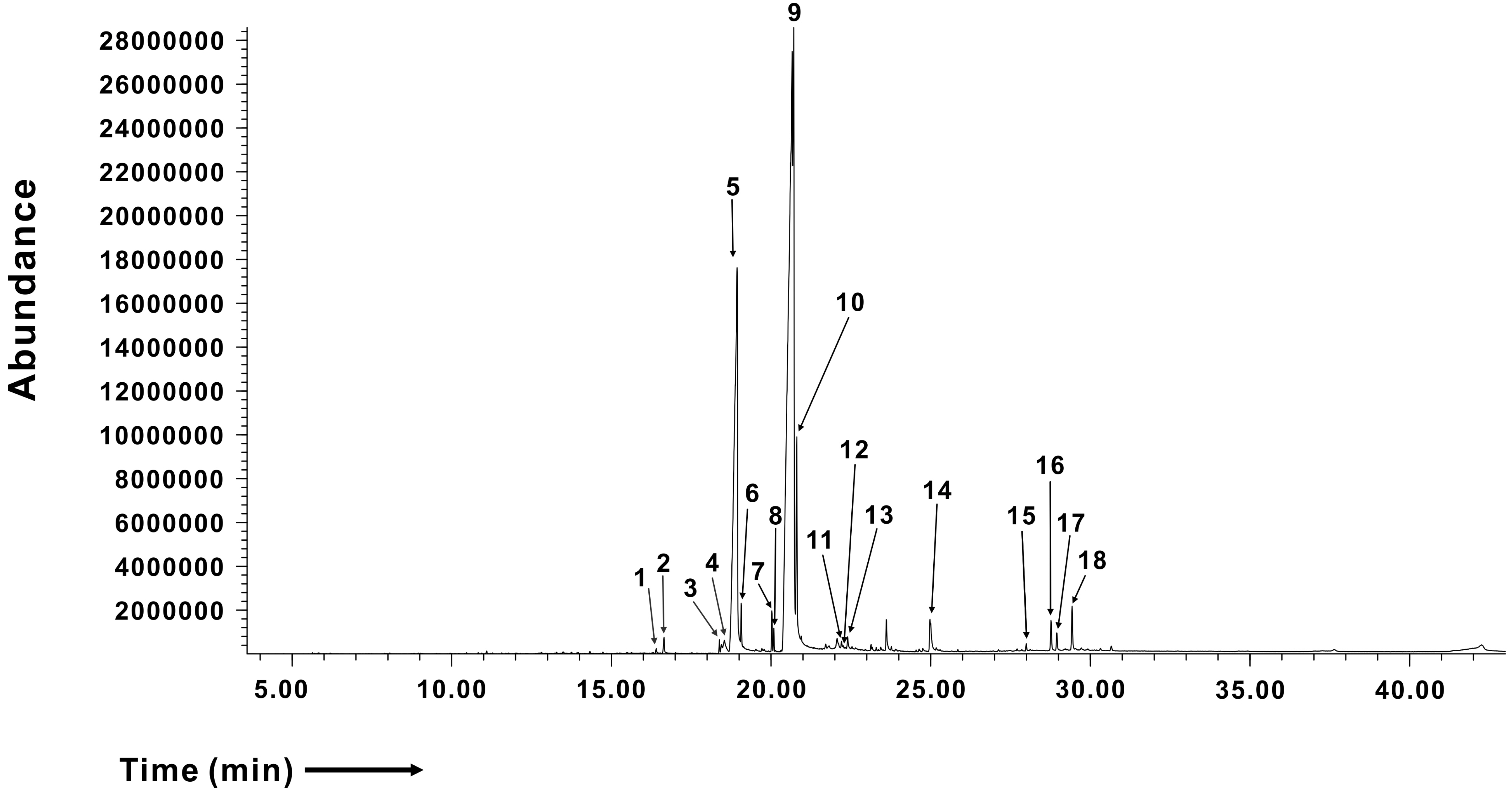
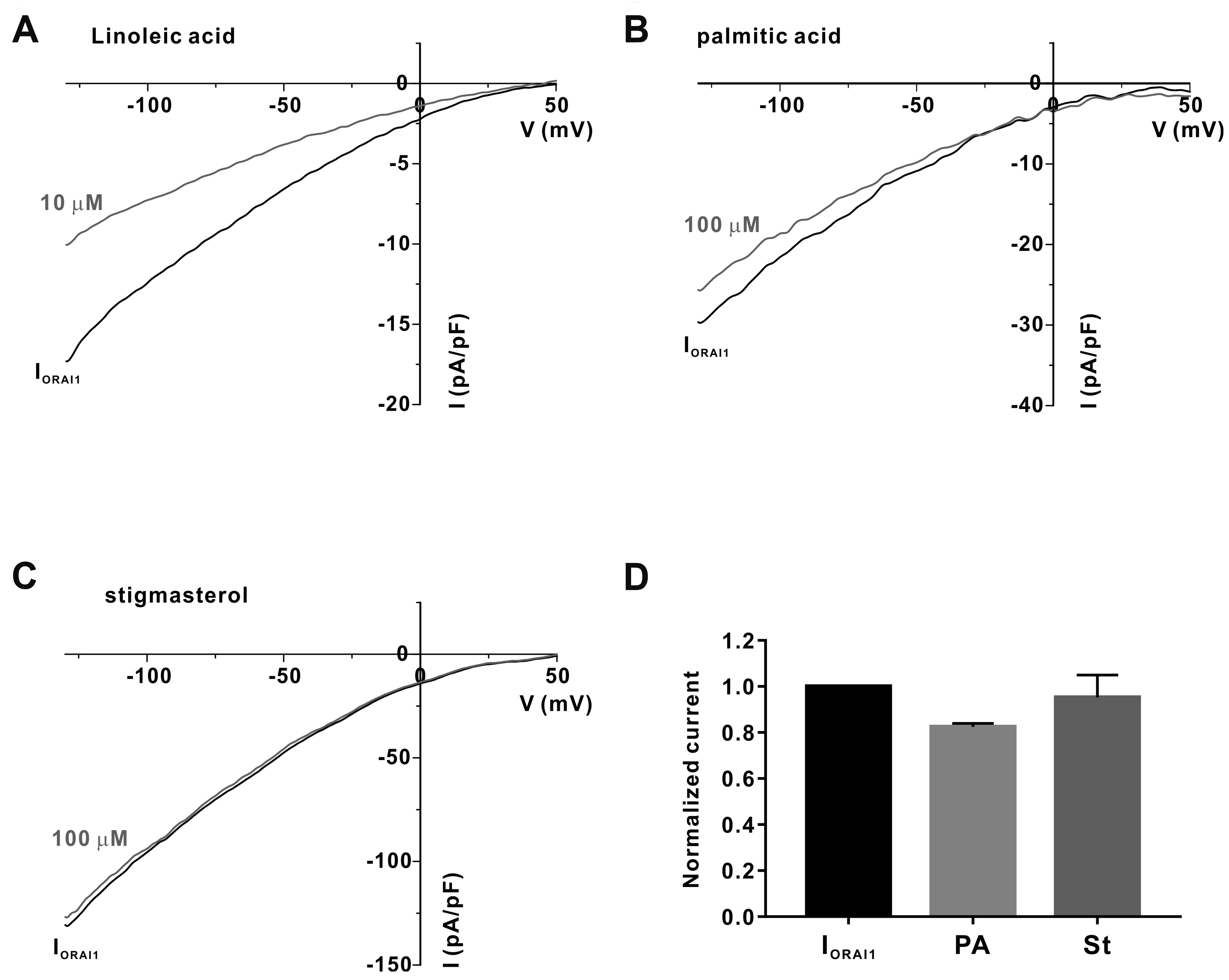
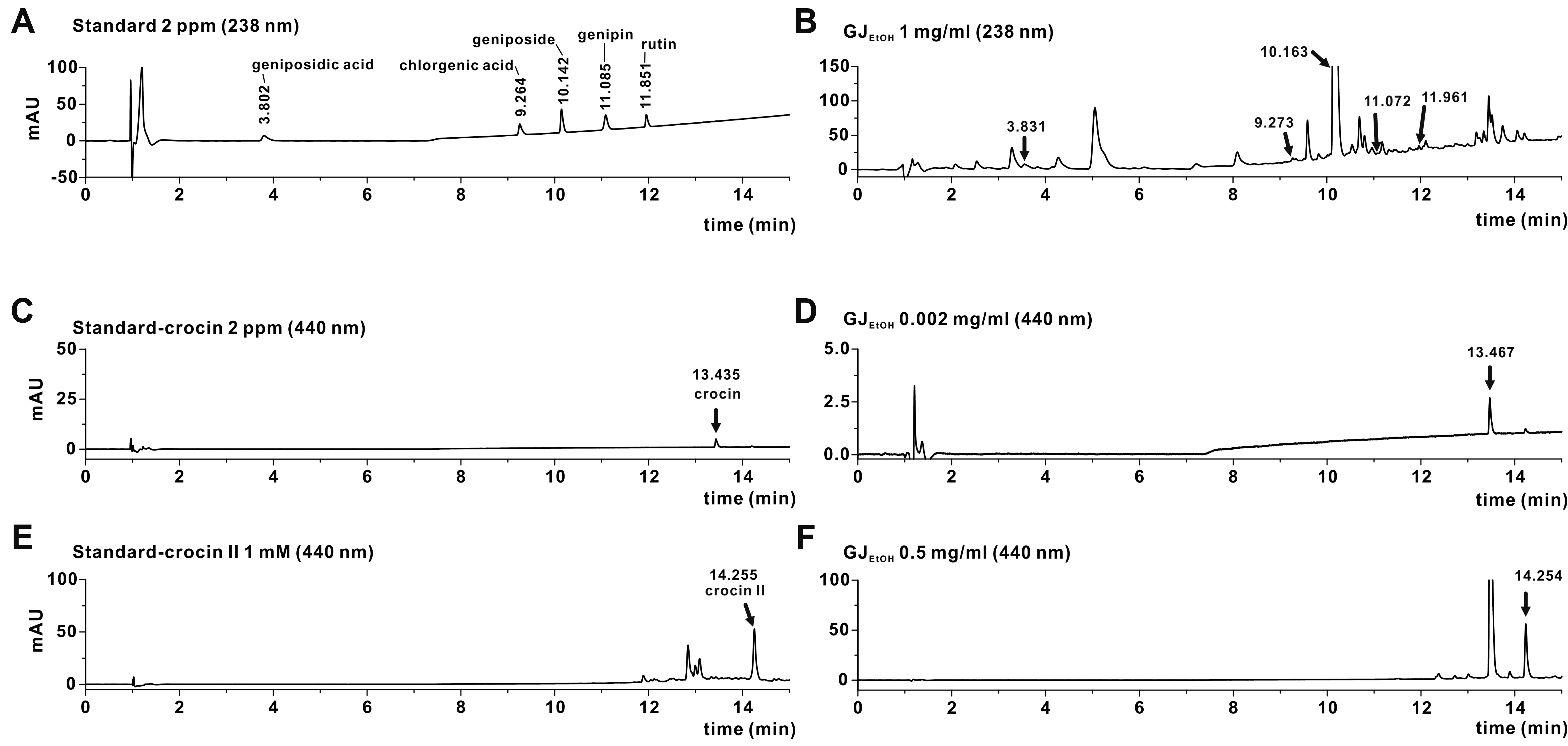
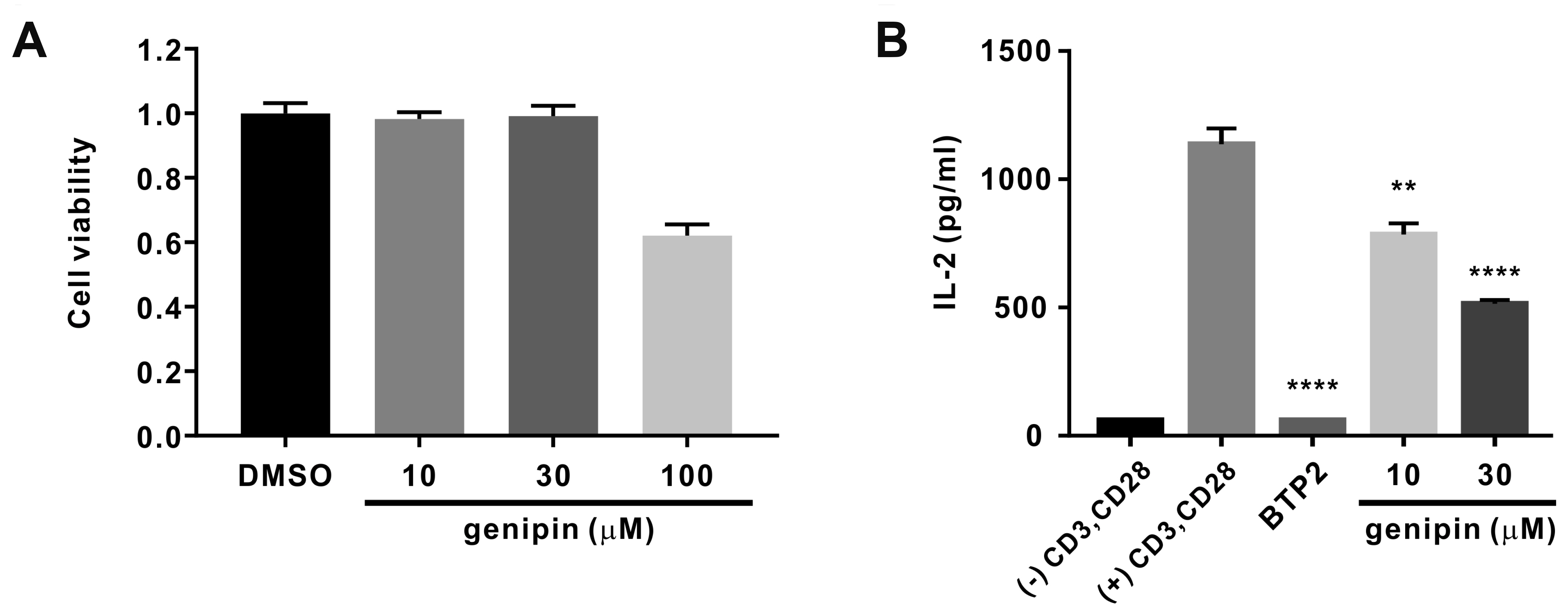
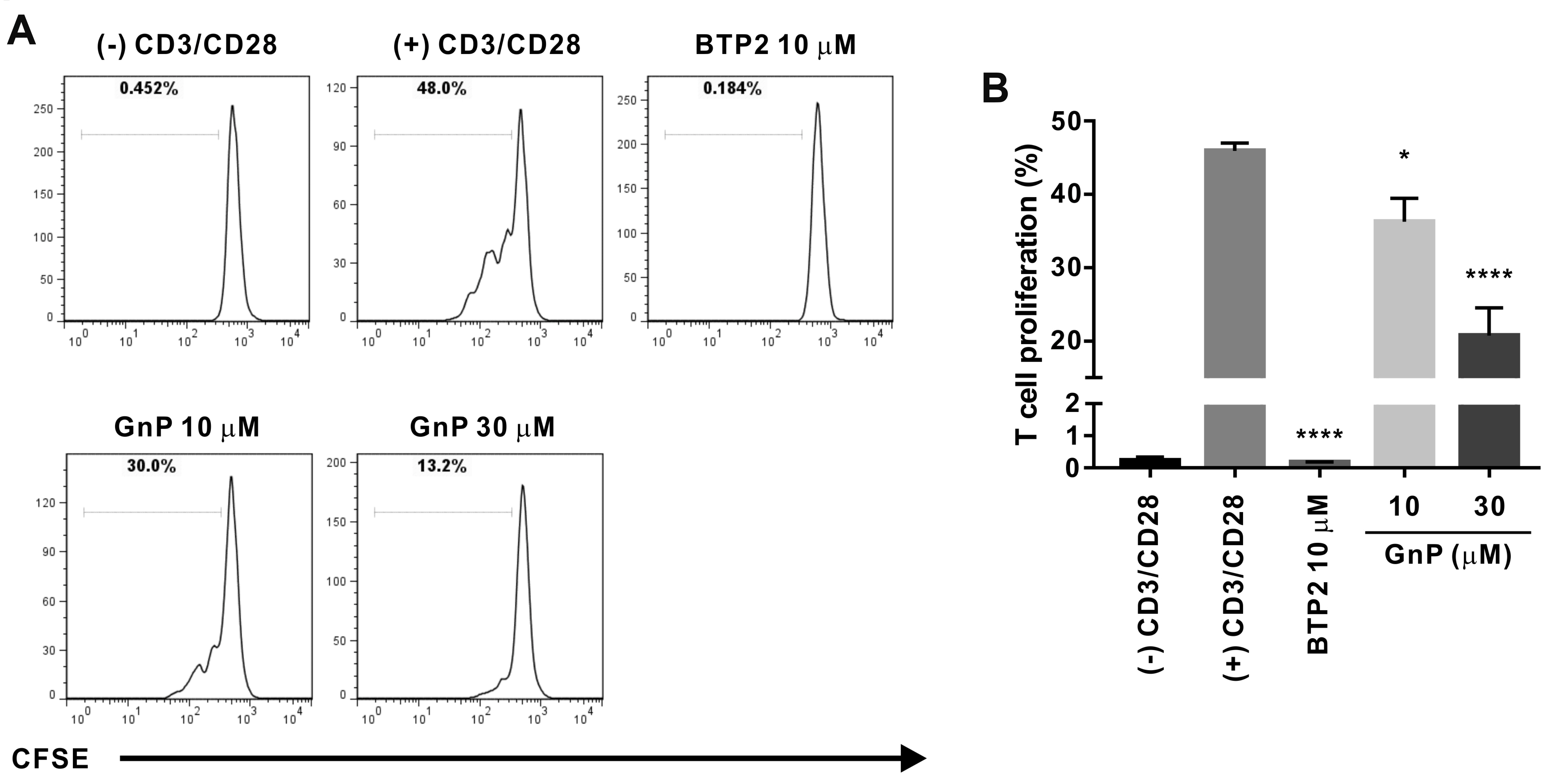
- Gardenia jasminoides (GJ) is a widely used herbal medicine with antiinflammatory properties, but its effects on the ORAI1 channel, which is important in generating intracellular calcium signaling for T cell activation, remain unknown. In this study, we investigated whether 70% ethanolic GJ extract (GJEtOH) and its subsequent fractions inhibit ORAI1 and determined which constituents contributed to this effect. Whole-cell patch clamp analysis revealed that GJEtOH (64.7% ± 3.83% inhibition at 0.1 mg/ml) and all its fractions showed inhibitory effects on the ORAI1 channel. Among the GJ fractions, the hexane fraction (GJHEX, 66.8% ± 9.95% at 0.1 mg/ml) had the most potent inhibitory effects in hORAI1-hSTIM1 co-transfected HEK293T cells. Chemical constituent analysis revealed that the strong ORAI1 inhibitory effect of GJHEX was due to linoleic acid, and in other fractions, we found that genipin inhibited ORAI1. Genipin significantly inhibited IORAI1 and interleukin-2 production in CD3/ CD28-stimulated Jurkat T lymphocytes by 35.9% ± 3.02% and 54.7% ± 1.32% at 30 μM, respectively. Furthermore, the same genipin concentration inhibited the proliferation of human primary CD4+ T lymphocytes stimulated with CD3/CD28 antibodies by 54.9% ± 8.22%, as evaluated by carboxyfluorescein succinimidyl ester assay. Our findings suggest that genipin may be one of the active components of GJ responsible for T cell suppression, which is partially mediated by activation of the ORAI1 channel. This study helps us understand the mechanisms of GJ in the treatment of inflammatory diseases.
Figure
Reference
-
1. Liu H, Chen YF, Li F, Zhang HY. 2013; Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J Asian Nat Prod Res. 15:94–110. DOI: 10.1080/10286020.2012.723203. PMID: 23211013.2. Deng Y, Guan M, Xie X, Yang X, Xiang H, Li H, Zou L, Wei J, Wang D, Deng X. 2013; Geniposide inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. Int Immunopharmacol. 17:561–567. DOI: 10.1016/j.intimp.2013.06.028. PMID: 23859870.
Article3. Sung YY, Lee AY, Kim HK. 2014; The Gardenia jasminoides extract and its constituent, geniposide, elicit anti-allergic effects on atopic dermatitis by inhibiting histamine in vitro and in vivo. J Ethnopharmacol. 156:33–40. DOI: 10.1016/j.jep.2014.07.060. PMID: 25153023.4. Koo HJ, Lim KH, Jung HJ, Park EH. 2006; Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 103:496–500. DOI: 10.1016/j.jep.2005.08.011. PMID: 16169698.
Article5. Xiao W, Li S, Wang S, Ho CT. 2017; Chemistry and bioactivity of Gardenia jasminoides. J Food Drug Anal. 25:43–61. DOI: 10.1016/j.jfda.2016.11.005. PMID: 28911543.6. Ko JW, Shin NR, Park SH, Cho YK, Kim JC, Seo CS, Shin IS. 2017; Genipin inhibits allergic responses in ovalbumin-induced asthmatic mice. Int Immunopharmacol. 53:49–55. DOI: 10.1016/j.intimp.2017.10.010. PMID: 29035815.
Article7. Nam JH, Kim WK. 2020; The role of TRP channels in allergic inflammation and its clinical relevance. Curr Med Chem. 27:1446–1468. DOI: 10.2174/0929867326666181126113015. PMID: 30474526.
Article8. Litosch I. 2016; Decoding Gαq signaling. Life Sci. 152:99–106. DOI: 10.1016/j.lfs.2016.03.037. PMID: 27012764.
Article9. Woo JS, Srikanth S, Gwack Y. Kozak JA, Putney JW, editors. 2018. Modulation of Orai1 and STIM1 by cellular factors. Calcium entry channels in non-excitable cells. CRC Press;Boca Raton (FL): p. 73–92. DOI: 10.1201/9781315152592-4. PMCID: PMC5369199.
Article10. Feske S, Wulff H, Skolnik EY. 2015; Ion channels in innate and adaptive immunity. Annu Rev Immunol. 33:291–353. DOI: 10.1146/annurev-immunol-032414-112212. PMID: 25861976. PMCID: PMC4822408.
Article11. Kim HJ, Nam YR, Kim EJ, Nam JH, Kim WK. 2018; Spirodela polyrhiza and its chemical constituent vitexin exert anti-allergic effect via ORAI1 channel inhibition. Am J Chin Med. 46:1243–1261. DOI: 10.1142/S0192415X18500659. PMID: 30149756.
Article12. Kim HJ, Woo J, Nam YR, Nam JH, Kim WK. 2019; Flos Magnoliae and its constituent linoleic acid suppress T lymphocyte activation via store-operated calcium entry. Am J Chin Med. 47:1627–1641. DOI: 10.1142/S0192415X19500836. PMID: 31659911.13. Lee S, Youn K, Jun M. 2018; Major compounds of red ginseng oil attenuate Aβ25-35-induced neuronal apoptosis and inflammation by modulating MAPK/NF-κB pathway. Food Funct. 9:4122–4134. DOI: 10.1039/C8FO00795K. PMID: 30014084.
Article14. Gabay O, Sanchez C, Salvat C, Chevy F, Breton M, Nourissat G, Wolf C, Jacques C, Berenbaum F. 2010; Stigmasterol: a phytosterol with potential anti-osteoarthritic properties. Osteoarthritis Cartilage. 18:106–116. DOI: 10.1016/j.joca.2009.08.019. PMID: 19786147.
Article15. Trickett A, Kwan YL. 2003; T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 275:251–255. DOI: 10.1016/S0022-1759(03)00010-3. PMID: 12667688.
Article16. Park SH, An JE, Jang S, Kim JY, Lee JW, Kim HK. 2019; Gardenia jasminoides extract without crocin improved atopic dermatitis-like skin lesions via suppression of Th2-related cytokines in Dfe-induced NC/Nga mice. J Ethnopharmacol. 241:112015. DOI: 10.1016/j.jep.2019.112015. PMID: 31173875.17. Sung YY, Kim HK. 2018; Crocin ameliorates atopic dermatitis symptoms by down regulation of Th2 response via blocking of NF-κB/STAT6 signaling pathways in mice. Nutrients. 10:1625. DOI: 10.3390/nu10111625. PMID: 30400140. PMCID: PMC6266819.
Article18. Srikanth S, Woo JS, Sun Z, Gwack Y. 2017; Immunological disorders: regulation of Ca2+ signaling in T lymphocytes. Adv Exp Med Biol. 993:397–424. DOI: 10.1007/978-3-319-57732-6_21. PMID: 28900926.19. Holowka D, Wilkes M, Stefan C, Baird B. 2016; Roles for Ca2+ mobilization and its regulation in mast cell functions: recent progress. Biochem Soc Trans. 44:505–509. DOI: 10.1042/BST20150273. PMID: 27068962. PMCID: PMC5293407.20. Shanmugam MK, Shen H, Tang FR, Arfuso F, Rajesh M, Wang L, Kumar AP, Bian J, Goh BC, Bishayee A, Sethi G. 2018; Potential role of genipin in cancer therapy. Pharmacol Res. 133:195–200. DOI: 10.1016/j.phrs.2018.05.007. PMID: 29758279.
Article21. Habtemariam S, Lentini G. 2018; Plant-derived anticancer agents: lessons from the pharmacology of geniposide and its aglycone, genipin. Biomedicines. 6:39. DOI: 10.3390/biomedicines6020039. PMID: 29587429. PMCID: PMC6027249.
Article22. Zhang Z, Wang X, Ma C, Li Z, Chen H, Zhang Z, Li T. 2019; Genipin protects rats against lipopolysaccharide-induced acute lung injury by reinforcing autophagy. Int Immunopharmacol. 72:21–30. DOI: 10.1016/j.intimp.2019.03.052. PMID: 30959368.
Article23. Yu SX, Du CT, Chen W, Lei QQ, Li N, Qi S, Zhang XJ, Hu GQ, Deng XM, Han WY, Yang YJ. 2015; Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci Rep. 5:17935. DOI: 10.1038/srep17935. PMID: 26659006. PMCID: PMC4675967.
Article24. Kim JS, Kim SJ, Lee SM. 2015; Genipin attenuates sepsis-induced immunosuppression through inhibition of T lymphocyte apoptosis. Int Immunopharmacol. 27:15–23. DOI: 10.1016/j.intimp.2015.04.034. PMID: 25921028.
Article25. Nam KN, Choi YS, Jung HJ, Park GH, Park JM, Moon SK, Cho KH, Kang C, Kang I, Oh MS, Lee EH. 2010; Genipin inhibits the inflammatory response of rat brain microglial cells. Int Immunopharmacol. 10:493–499. DOI: 10.1016/j.intimp.2010.01.011. PMID: 20123040.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation and identification of the yeasts from sputum or other clinical specimens using the medium containing pigments extract of gardenia jasminoides fruits
- Antitumor Effects of Genipin: New and Emerging Insights from Recent Studies
- Genipin Inhibits Hypoxia-Induced Accumulation of HIF-1α and VEGF Expressions in Human Cervical Carcinoma Cells
- Alterations in the Kinetics of CD4+ T Cell Responses with Aging
- Flos magnoliae constituent fargesin has an anti-allergic effect via ORAI1 channel inhibition