Korean J Physiol Pharmacol.
2021 May;25(3):251-258. 10.4196/kjpp.2021.25.3.251.
Flos magnoliae constituent fargesin has an anti-allergic effect via ORAI1 channel inhibition
- Affiliations
-
- 1Department of Physiology, Dongguk University College of Medicine, Gyeongju 38066, Korea
- 2Channelopathy Research Center (CRC), Dongguk University College of Medicine, Goyang 10326, Korea
- 3Department of Internal Medicine, Graduate School of Medicine, Dongguk University, Goyang 10326, Korea
- KMID: 2514950
- DOI: http://doi.org/10.4196/kjpp.2021.25.3.251
Abstract
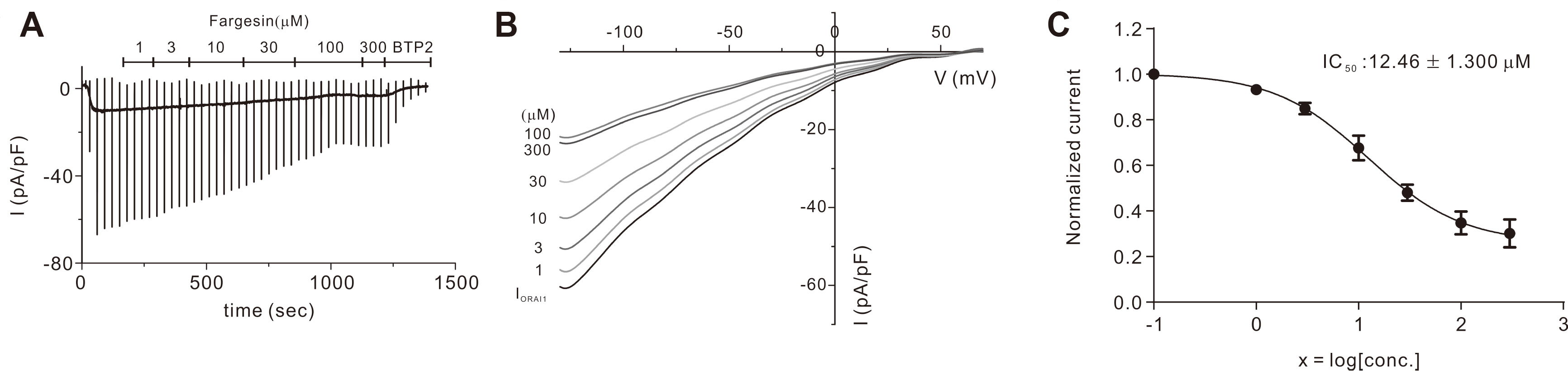
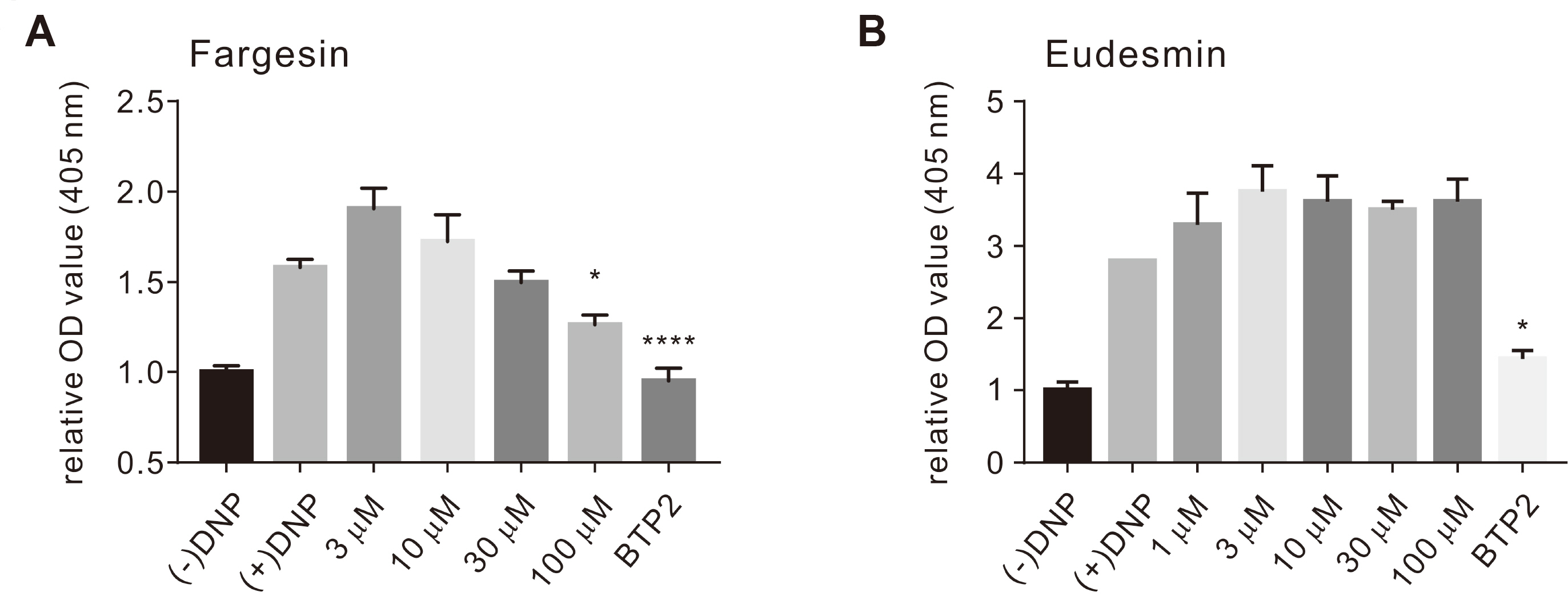
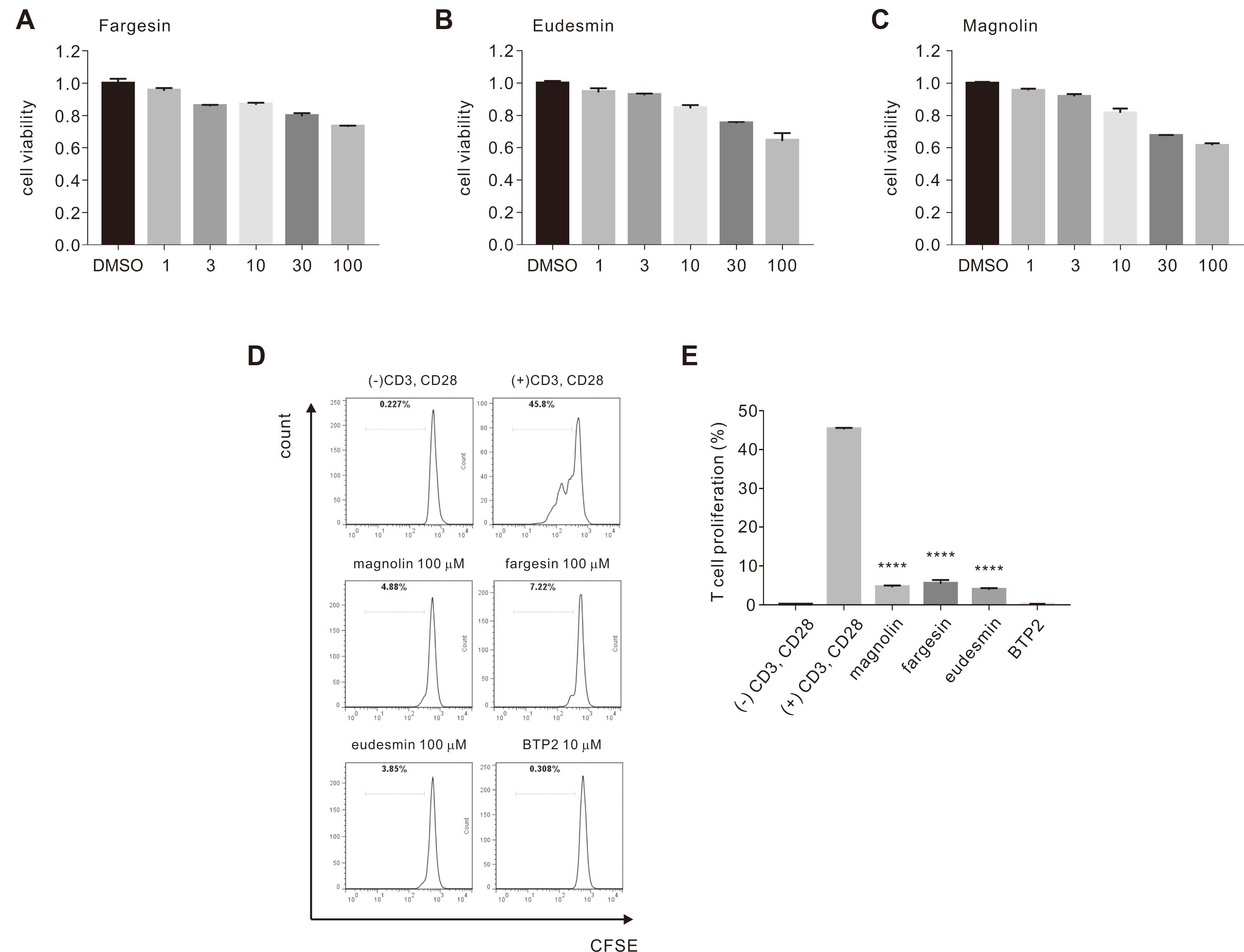
- Flos magnoliae (FM), the dry flower buds of Magnolia officinalis or its related species, is a traditional herbal medicine commonly used in Asia for symptomatic relief of and treating allergic rhinitis, headache, and sinusitis. Although several studies have reported the effects of FM on store-operated calcium entry (SOCE) via the ORAI1 channel, which is essential during intracellular calcium signaling cascade generation for T cell activation and mast cell degranulation, the effects of its isolated constituents on SOCE remain unidentified. Therefore, we investigated which of the five major constituents of 30% ethanoic FM (vanillic acid, tiliroside, eudesmin, magnolin, and fargesin) inhibit SOCE and their physiological effects on immune cells. The conventional whole-cell patch clamp results showed that fargesin, magnolin, and eudesmin significantly inhibited SOCE and thus human primary CD4 + T lymphocyte proliferation, as well as allergen-induced histamine release in mast cells. Among them, fargesin demonstrated the most potent inhibitory effects not only on ORAI1 (IC 50 = 12.46 ± 1.300 μM) but also on T-cell proliferation (by 87.74% ± 1.835%) and mast cell degranulation (by 20.11% ± 5.366%) at 100 μM. Our findings suggest that fargesin can be a promising candidate for the development of therapeutic drugs to treat allergic diseases.
Keyword
Figure
Reference
-
1. Junttila IS. 2018; Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. 9:888. DOI: 10.3389/fimmu.2018.00888. PMID: 29930549. PMCID: PMC6001902.
Article2. Rajan TV. 2003; The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 24:376–379. DOI: 10.1016/S1471-4906(03)00142-X. PMID: 12860528.
Article3. Galli SJ, Tsai M, Piliponsky AM. 2008; The development of allergic inflammation. Nature. 454:445–454. DOI: 10.1038/nature07204. PMID: 18650915. PMCID: PMC3573758.
Article4. Chassin H, Geering B, Schukur L, Ausländer D, Lang B, Fussenegger M. 2017; Sensing and responding to allergic response cytokines through a genetically encoded circuit. Nat Commun. 8:1101. DOI: 10.1038/s41467-017-01211-1. PMID: 29062109. PMCID: PMC5653676.
Article5. Ozdemir C, Akdis M, Akdis CA. 2008; Nature of regulatory T cells in the context of allergic disease. Allergy Asthma Clin Immunol. 4:106–110. DOI: 10.1186/1710-1492-4-3-106. PMID: 20525131. PMCID: PMC2868864.
Article6. Lenon GB, Xue CC, Story DF, Thien FC, McPhee S, Li CG. 2007; Inhibition of release of inflammatory mediators in primary and cultured cells by a Chinese herbal medicine formula for allergic rhinitis. Chin Med. 2:2. DOI: 10.1093/ecam/nel083. PMID: 17549238. PMCID: PMC1876611.7. Izquierdo JH, Bonilla-Abadía F, Cañas CA, Tobón GJ. 2014; Calcium, channels, intracellular signaling and autoimmunity. Reumatol Clin. 10:43–47. DOI: 10.1016/j.reumae.2013.11.004. PMID: 24001934.
Article8. Ma HT, Beaven MA. 2009; Regulation of Ca2+ signaling with particular focus on mast cells. Crit Rev Immunol. 29:155–186. DOI: 10.1615/CritRevImmunol.v29.i2.40. PMID: 19496745. PMCID: PMC2954050.9. Feske S, Wulff H, Skolnik EY. 2015; Ion channels in innate and adaptive immunity. Annu Rev Immunol. 33:291–353. DOI: 10.1146/annurev-immunol-032414-112212. PMID: 25861976. PMCID: PMC4822408.
Article10. Feske S. 2019; CRAC channels and disease - from human CRAC channelopathies and animal models to novel drugs. Cell Calcium. 80:112–116. DOI: 10.1016/j.ceca.2019.03.004. PMID: 31009822. PMCID: PMC6545165.
Article11. Holgate ST, Broide D. 2003; New targets for allergic rhinitis--a disease of civilization. Nat Rev Drug Discov. 2:902–914. DOI: 10.1038/nrd1224. PMID: 14668811.12. Bagur R, Hajnóczky G. 2017; Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol Cell. 66:780–788. DOI: 10.1016/j.molcel.2017.05.028. PMID: 28622523. PMCID: PMC5657234.13. Yang PC, Jafri MS. 2020; Ca2+ signaling in T lymphocytes: the interplay of the endoplasmic reticulum, mitochondria, membrane potential, and CRAC channels on transcription factor activation. Heliyon. 6:e03526. DOI: 10.1016/j.heliyon.2020.e03526. PMID: 32181396. PMCID: PMC7063158.14. Jafri MS, Keizer J. 1995; On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophys J. 69:2139–2153. DOI: 10.1016/S0006-3495(95)80088-3. PMID: 8580358. PMCID: PMC1236448.15. Vaeth M, Kahlfuss S, Feske S. 2020; CRAC channels and calcium signaling in T cell-mediated immunity. Trends Immunol. 41:878–901. DOI: 10.1016/j.it.2020.06.012. PMID: 32711944. PMCID: PMC7985820.
Article16. Feske S. 2009; Calcium signals in lymphocyte activation and disease. Cell Commun Signal. 7(Suppl 1):A76. DOI: 10.1186/1478-811X-7-S1-A76. PMCID: PMC4291824.
Article17. Prakriya M, Lewis RS. 2015; Store-operated calcium channels. Physiol Rev. 95:1383–1436. DOI: 10.1152/physrev.00020.2014. PMID: 26400989. PMCID: PMC4600950.
Article18. McCarl CA, Picard C, Khalil S, Kawasaki T, Röther J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S. 2009; ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 124:1311–1318.e7. DOI: 10.1016/j.jaci.2009.10.007. PMID: 20004786. PMCID: PMC2829767.19. Yan S, Chen W, Zhang Y, Li J, Chen X. 2019; Calcium release-activated calcium modulator 1 as a therapeutic target in allergic skin diseases. Life Sci. 228:152–157. DOI: 10.1016/j.lfs.2019.05.001. PMID: 31055088.
Article20. Aki A, Tanaka K, Nagaoka N, Kimura T, Baba D, Onodera Y, Wada T, Maeda H, Nakanishi T, Agatsuma T, Komai T. 2020; Anti-ORAI1 antibody DS-2741a, a specific CRAC channel blocker, shows ideal therapeutic profiles for allergic disease via suppression of aberrant T-cell and mast cell activation. FASEB Bioadv. 2:478–488. DOI: 10.1096/fba.2020-00008. PMID: 32821879. PMCID: PMC7429349.
Article21. Kim HJ, Nam YR, Nam JH. 2018; Flos Magnoliae inhibits chloride secretion via ANO1 inhibition in Calu-3 cells. Am J Chin Med. 46:1079–1092. DOI: 10.1142/S0192415X18500568. PMID: 29976084.
Article22. Kim HJ, Woo J, Nam YR, Nam JH, Kim WK. 2019; Flos Magnoliae and its constituent linoleic acid suppress T lymphocyte activation via store-operated calcium entry. Am J Chin Med. 47:1627–1641. DOI: 10.1142/S0192415X19500836. PMID: 31659911.23. Shen Y, Pang EC, Xue CC, Zhao ZZ, Lin JG, Li CG. 2008; Inhibitions of mast cell-derived histamine release by different Flos Magnoliae species in rat peritoneal mast cells. Phytomedicine. 15:808–814. DOI: 10.1016/j.phymed.2008.04.012. PMID: 18585022.
Article24. Nguyen TTM, Lee HS, Nguyen TT, Ngo TQM, Jun CD, Min BS, Kim JA. 2017; Four new lignans and IL-2 inhibitors from Magnoliae Flos. Chem Pharm Bull (Tokyo). 65:840–847. DOI: 10.1248/cpb.c17-00314. PMID: 28867711.
Article25. Shin TY, Kim DK, Chae BS, Lee EJ. 2001; Antiallergic action of Magnolia officinalis on immediate hypersensitivity reaction. Arch Pharm Res. 24:249–255. DOI: 10.1007/BF02978266. PMID: 11440086.
Article26. Nam JH, Jung HW, Chin YW, Yang WM, Bae HS, Kim WK. 2017; Spirodela polyrhiza extract modulates the activation of atopic dermatitis-related ion channels, Orai1 and TRPV3, and inhibits mast cell degranulation. Pharm Biol. 55:1324–1329. DOI: 10.1080/13880209.2017.1300819. PMID: 28290212. PMCID: PMC6130684.
Article27. Kim HJ, Nam YR, Kim EJ, Nam JH, Kim WK. 2018; Spirodela polyrhiza and its chemical constituent vitexin exert anti-allergic effect via ORAI1 channel inhibition. Am J Chin Med. 46:1243–1261. DOI: 10.1142/S0192415X18500659. PMID: 30149756.
Article28. Woo JH, Nam DY, Kim HJ, Hong PTL, Kim WK, Nam JH. 2021; Nootkatol prevents ultraviolet radiation-induced photoaging via ORAI1 and TRPV1 inhibition in melanocytes and keratinocytes. Korean J Physiol Pharmacol. 25:87–94. DOI: 10.4196/kjpp.2021.25.1.87. PMID: 33361541. PMCID: PMC7756533.
Article29. Nam JH, Lee DU. 2016; Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J Dermatol Sci. 84:305–313. DOI: 10.1016/j.jdermsci.2016.09.017. PMID: 27712859.
Article30. Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. 2008; Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 9:89–96. DOI: 10.1038/ni1550. PMID: 18059270. PMCID: PMC2377025.
Article31. Ma P, Che D, Zhao T, Zhang Y, Li C, An H, Zhang T, He H. 2019; Magnolin inhibits IgE/Ag-induced allergy in vivo and in vitro. Int Immunopharmacol. 76:105867. DOI: 10.1016/j.intimp.2019.105867. PMID: 31520994.
Article32. Liang Y, Zhang X, Zou J, Shi Y, Wang Y, Tai J, Yang Y, Zhou X, Guo D, Wang J, Cheng J, Yang M. 2019; Pharmacology mechanism of Flos magnoliae and Centipeda minima for treating allergic rhinitis based on pharmacology network. Drug Dev Ind Pharm. 45:1547–1555. DOI: 10.1080/03639045.2019.1635150. PMID: 31216904.33. Jung YS, Weon JB, Yang WS, Ryu G, Ma CJ. 2018; Neuroprotective effects of Magnoliae Flos extract in mouse hippocampal neuronal cells. Sci Rep. 8:9693. DOI: 10.1038/s41598-018-28055-z. PMID: 29946137. PMCID: PMC6018738.
Article34. Kobayashi S, Kobayashi H, Matsuno H, Kimura I, Kimura M. 1998; Inhibitory effects of anti-rheumatic drugs containing magnosalin, a compound from 'Shin-i' (Flos magnoliae), on the proliferation of synovial cells in rheumatoid arthritis models. Immunopharmacology. 39:139–147. DOI: 10.1016/S0162-3109(98)00004-6. PMID: 9716260.
Article35. Kim GC, Lee SG, Park BS, Kim JY, Song YS, Kim JM, Yoo KS, Huh GY, Jeong MH, Lim YJ, Kim HM, Yoo YH. 2003; Magnoliae flos induces apoptosis of RBL-2H3 cells via mitochondria and caspase. Int Arch Allergy Immunol. 131:101–110. DOI: 10.1159/000070925. PMID: 12811018.
Article36. Galli SJ, Gaudenzio N, Tsai M. 2020; Mast cells in inflammation and disease: recent progress and ongoing concerns. Annu Rev Immunol. 38:49–77. DOI: 10.1146/annurev-immunol-071719-094903. PMID: 32340580.
Article37. Pham TH, Kim MS, Le MQ, Song YS, Bak Y, Ryu HW, Oh SR, Yoon DY. 2017; Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-ĸB signaling. Phytomedicine. 24:96–103. DOI: 10.1016/j.phymed.2016.11.014. PMID: 28160867.
Article38. Yue B, Ren YJ, Zhang JJ, Luo XP, Yu ZL, Ren GY, Sun AN, Deng C, Wang ZT, Dou W. 2018; Anti-inflammatory effects of fargesin on chemically induced inflammatory bowel disease in mice. Molecules. 23:1380. DOI: 10.3390/molecules23061380. PMID: 29880739. PMCID: PMC6100621.
Article39. Wang X, Cheng Y, Xue H, Yue Y, Zhang W, Li X. 2015; Fargesin as a potential β₁ adrenergic receptor antagonist protects the hearts against ischemia/reperfusion injury in rats via attenuating oxidative stress and apoptosis. Fitoterapia. 105:16–25. DOI: 10.1016/j.fitote.2015.05.016. PMID: 26025856.40. Fu T, Chai B, Shi Y, Dang Y, Ye X. 2019; Fargesin inhibits melanin synthesis in murine malignant and immortalized melanocytes by regulating PKA/CREB and P38/MAPK signaling pathways. J Dermatol Sci. 94:213–219. DOI: 10.1016/j.jdermsci.2019.03.004. PMID: 30956031.
Article41. Wang G, Gao JH, He LH, Yu XH, Zhao ZW, Zou J, Wen FJ, Zhou L, Wan XJ, Tang CK. 2020; Fargesin alleviates atherosclerosis by promoting reverse cholesterol transport and reducing inflammatory response. Biochim Biophys Acta Mol Cell Biol Lipids. 1865:158633. DOI: 10.1016/j.bbalip.2020.158633. PMID: 31988050.
Article42. Lim H, Son KH, Bae KH, Hung TM, Kim YS, Kim HP. 2009; 5-lipoxygenase-inhibitory constituents from Schizandra fructus and Magnolia flos. Phytother Res. 23:1489–1492. DOI: 10.1002/ptr.2783. PMID: 19277963.
Article43. Zhang X, Qian F, Tan JJ, Guo FJ, Kulka M, Xu JW, Li YM. 2017; Bioassay-guided isolation of bisepoxylignans from the flower buds of Magnolia biondii Pamp and their antiallergic effects. RSC Advances. 7:34236–34243. DOI: 10.1039/C7RA01476G.
Article44. Lin Y, Xu J, Jia Q, Sun W, Fu J, Lv Y, Han S. 2020; Cell membrane chromatography coupled online with LC-MS to screen anti-anaphylactoid components from Magnolia biondii Pamp. targeting on Mas-related G protein-coupled receptor X2. J Sep Sci. 43:2571–2578. DOI: 10.1002/jssc.202000014. PMID: 32281296.45. Lee CJ, Lee MH, Yoo SM, Choi KI, Song JH, Jang JH, Oh SR, Ryu HW, Lee HS, Surh YJ, Cho YY. 2015; Magnolin inhibits cell migration and invasion by targeting the ERKs/RSK2 signaling pathway. BMC Cancer. 15:576. DOI: 10.1186/s12885-015-1580-7. PMID: 26253302. PMCID: PMC4529708.
Article46. Wang J, Zhang S, Huang K, Shi L, Zhang Q. 2020; Magnolin inhibits proliferation and invasion of breast cancer MDA-MB-231 cells by targeting the ERK1/2 signaling pathway. Chem Pharm Bull (Tokyo). 68:421–427. DOI: 10.1248/cpb.c19-00820. PMID: 32378540.
Article47. Cho JY, Yoo ES, Baik KU, Park MH. 1999; Eudesmin inhibits tumor necrosis factor-alpha production and T cell proliferation. Arch Pharm Res. 22:348–353. DOI: 10.1007/BF02979056. PMID: 10489872.48. Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. 2008; Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A. 105:2011–2016. DOI: 10.1073/pnas.0706122105. PMID: 18250319. PMCID: PMC2538873.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gardenia jasminoides extract and its constituent, genipin, inhibit activation of CD3/CD28 co-stimulated CD4+ T cells via ORAI1 channel
- Downregulation of Orai1 expression in the airway alleviates murine allergic rhinitis
- Clinical and pathological significance of Orai1 channel expression in human diabetic nephropathy
- Involvement of Orai1 in tunicamycin-induced endothelial dysfunction
- Screening of Anti-HIV-1 Activity of Natural Product by MTT Assay