J Korean Neurosurg Soc.
2020 May;63(3):314-320. 10.3340/jkns.2020.0052.
Secondary Neurulation Defects-1 : Retained Medullary Cord
- Affiliations
-
- 1Division of Pediatric Neurosurgery, Seoul National University Children's Hospital, Seoul, Korea
- 2Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2501717
- DOI: http://doi.org/10.3340/jkns.2020.0052
Abstract
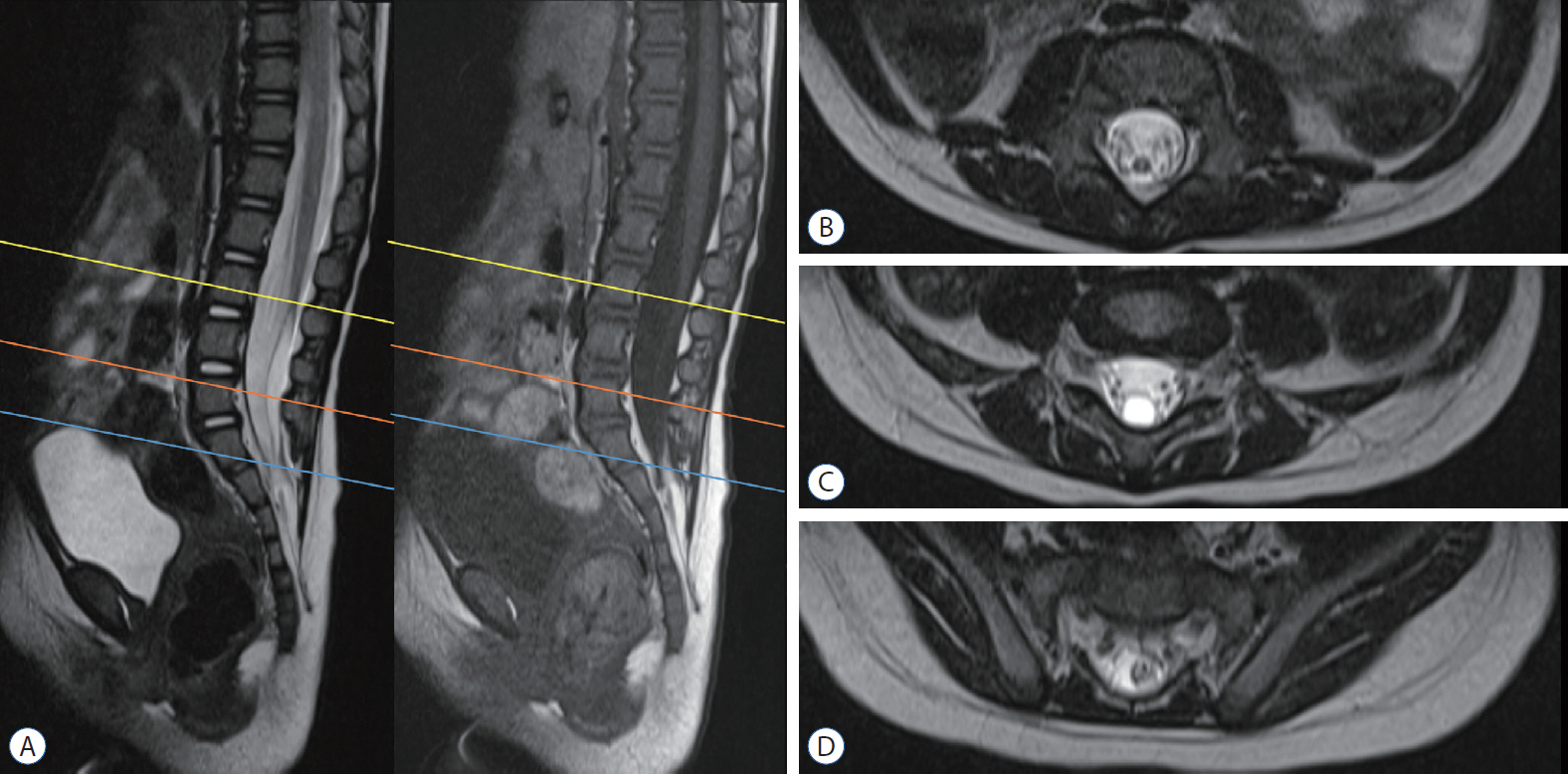
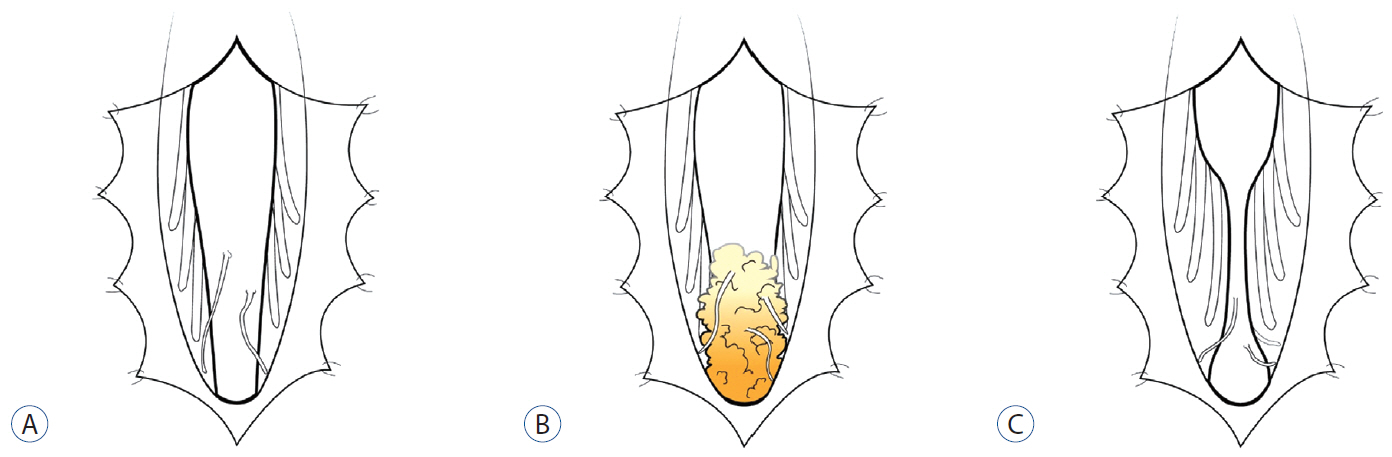
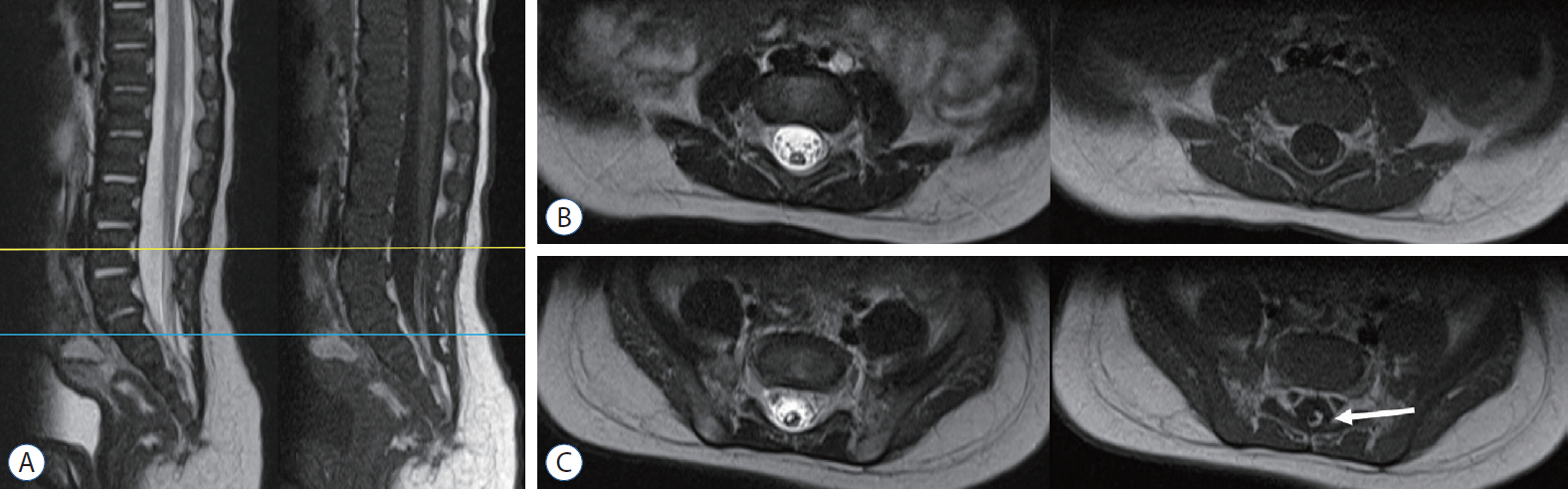
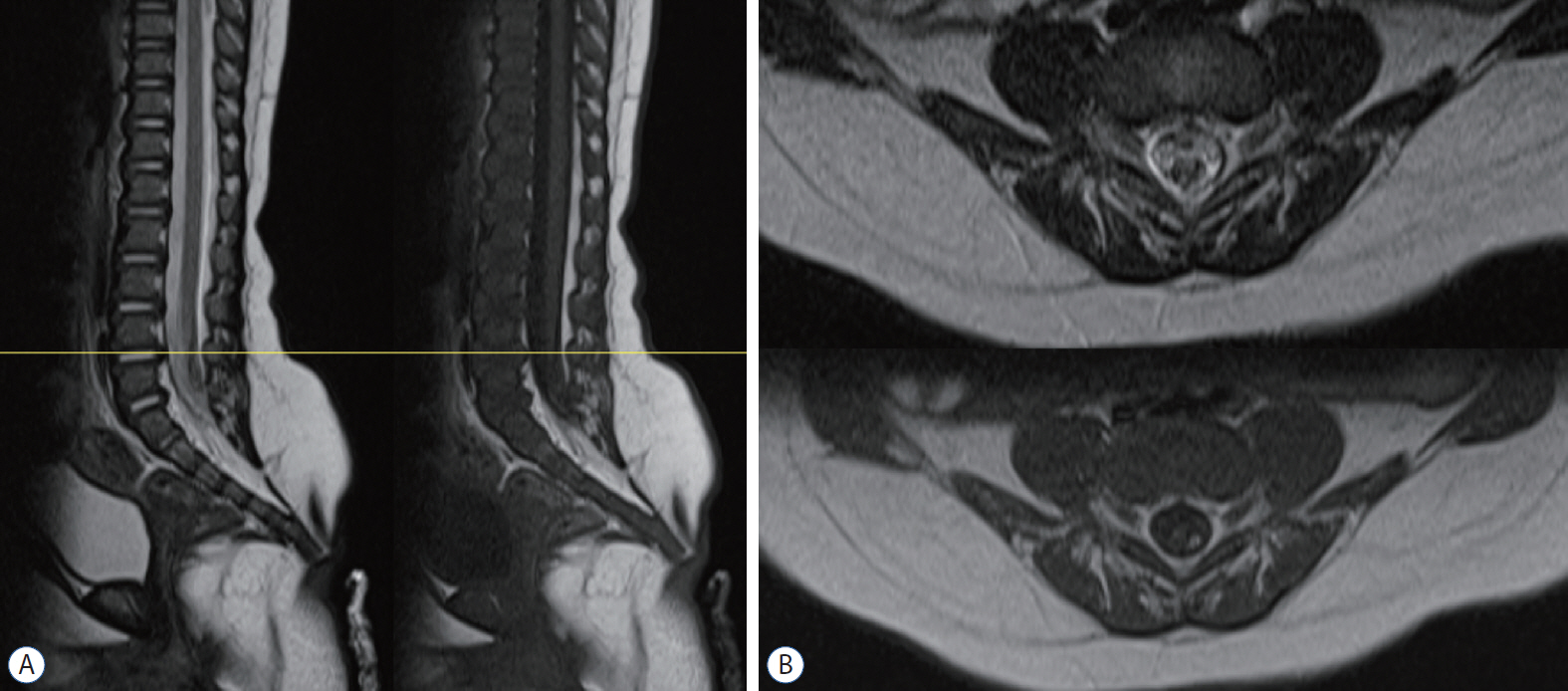
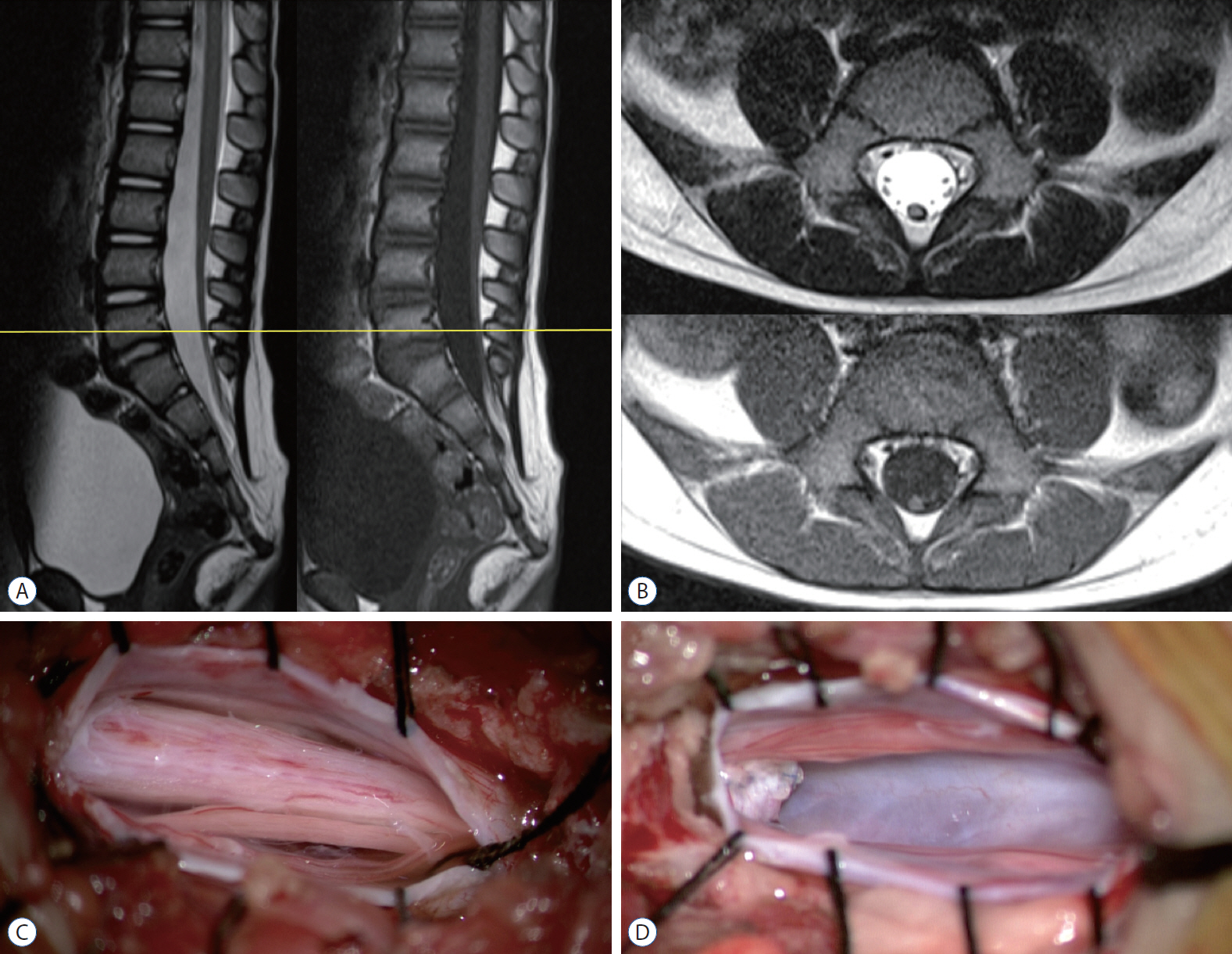
- Retained medullary cord (RMC) is a relatively recent term. Pang et al. newly defined the RMC as a late arrest of secondary neurulation leaving a non-functional vestigial portion at the tip of the conus medullaris. RMC, which belongs to the category of closed spinal dysraphism, is a cord-like structure that is elongated from the conus toward the cul-de-sac. Because intraoperative electrophysiological confirmation of a non-functional conus is essential for the diagnosis of RMC, only a tentative or an assumptive diagnosis is possible before surgery or in cases of limited surgical exposure. We suggest the term ‘possible RMC’ for these cases. An RMC may cause tethered cord syndrome and thus requires surgery. This article reviews the literature to elucidate the pathoembryogenesis, clinical significance and treatment of RMCs.
Keyword
Figure
Cited by 2 articles
-
Disorders of Secondary Neurulation : Mainly Focused on Pathoembryogenesis
Jeyul Yang, Ji Yeoun Lee, Kyung Hyun Kim, Kyu-Chang Wang
J Korean Neurosurg Soc. 2021;64(3):386-405. doi: 10.3340/jkns.2021.0023.Perspectives : The Role of Clinicians in Understanding Secondary Neurulation
Kyu-Chang Wang
J Korean Neurosurg Soc. 2021;64(3):414-417. doi: 10.3340/jkns.2021.0040.
Reference
-
References
1. Borner C, Monney L, Olivier R, Rossé T, Häcki J, Conus S. Life and death in a medieval atmosphere. Cell Death Differ. 6:201–206. 1999.
Article2. Chung YN, Lee DH, Yang HJ, Kim SK, Lee YJ, Lee MS, et al. Expression of neuronal markers in the secondary neurulation of chick embryos. Childs Nerv Syst. 24:105–110. 2008.
Article3. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 35:495–516. 2007.
Article4. Griffith CM, Wiley MJ, Sanders EJ. The vertebrate tail bud: three germ layers from one tissue. Anat Embryol (Berl). 185:101–113. 1992.
Article5. Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 279:300–307. 1998.6. Hughes AF, Freeman RB. Comparative remarks on the development of the tail cord among higher vertebrates. J Embryol Exp Morphol. 32:355–363. 1974.
Article7. Mills CL, Bellairs R. Mitosis and cell death in the tail of the chick embryo. Anat Embryol (Berl). 180:301–308. 1989.
Article8. Morioka T, Murakami N, Kanata A, Tsukamoto H, Suzuki SO. Retained medullary cord with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst. 36:423–427. 2020.
Article9. Müller F, O'Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat Embryol (Berl). 176:413–430. 1987.
Article10. Müller F, O'Rahilly R. The primitive streak, the caudal eminence and related structures in staged human embryos. Cells Tissues Organs. 177:2–20. 2004.
Article11. Murakami N, Morioka T, Shimogawa T, Hashiguchi K, Mukae N, Uchihashi K, et al. Retained medullary cord extending to a sacral subcutaneous meningocele. Childs Nerv Syst. 34:527–533. 2018.
Article12. Nievelstein RA, Hartwig NG, Vermeij-Keers C, Valk J. Embryonic development of the mammalian caudal neural tube. Teratology. 48:21–31. 1993.
Article13. Nijhawan D, Honarpour N, Wang X. Apoptosis in neural development and disease. Annu Rev Neurosci. 23:73–87. 2000.
Article14. O'Rahilly R, Müller F. Neurulation in the normal human embryo. Ciba Found Symp. 181:70–82. discussion 82-89. 1994.15. Pang D, Chong S, Wang KC. Secondary Neurulation Defects-1: Thickened Filum Terminale, Retained Medullary Cord. In : Di Rocco C, Pang D, Rutka J, editors. Textbook of Pediatric Neurosurgery. Cham: Springer;2017. p. 1–18.16. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: late arrest of secondary neurulation. Neurosurgery. 68:1500–1519. discussion 1519. 2011.
Article17. Sala F, Barone G, Tramontano V, Gallo P, Ghimenton C. Retained medullary cord confirmed by intraoperative neurophysiological mapping. Childs Nerv Syst. 30:1287–1291. 2014.
Article18. Schoenwolf GC. Morphogenetic processes involved in the remodeling of the tail region of the chick embryo. Anat Embryol (Berl). 162:183–197. 1981.
Article19. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 34:1785–1790. 2018.
Article20. Yang HJ, Wang KC, Chi JG, Lee MS, Lee YJ, Kim SK, et al. Cytokinetics of secondary neurulation in chick embryos: Hamburger and Hamilton stages 16-45. Childs Nerv Syst. 22:567–571. 2006.
Article21. Yang HJ, Wang KC, Chi JG, Lee MS, Lee YJ, Kim SK, et al. Neural differentiation of caudal cell mass (secondary neurulation) in chick embryos: Hamburger and Hamilton stages 16-45. Brain Res Dev Brain Res. 142:31–36. 2003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disorders of Secondary Neurulation : Mainly Focused on Pathoembryogenesis
- Perspectives : The Role of Clinicians in Understanding Secondary Neurulation
- Overview of Secondary Neurulation
- Spinal Dysraphism in the Last Two Decades : What I Have Seen during the Era of Dynamic Advancement
- Transcriptional Signature of Valproic Acid-Induced Neural Tube Defects in Human Spinal Cord Organoids