Int J Stem Cells.
2023 Nov;16(4):385-393. 10.15283/ijsc23012.
Transcriptional Signature of Valproic Acid-Induced Neural Tube Defects in Human Spinal Cord Organoids
- Affiliations
-
- 1Department of Anatomy, Brain Korea 21 Plus Program for Biomedical Science, Korea University College of Medicine, Seoul, Korea
- KMID: 2548002
- DOI: http://doi.org/10.15283/ijsc23012
Abstract
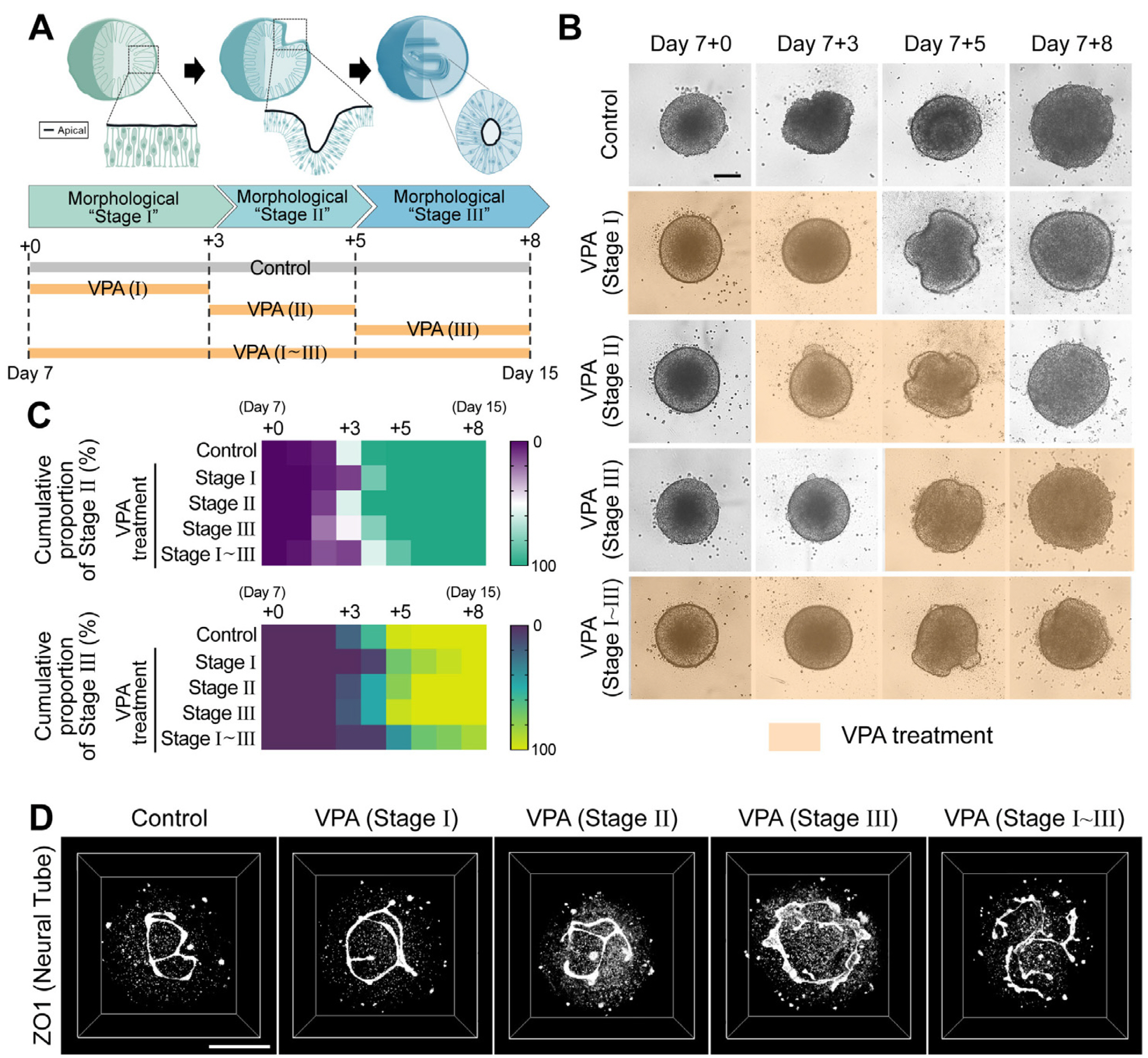
- In vertebrates, the entire central nervous system is derived from the neural tube, which is formed through a conserved early developmental morphogenetic process called neurulation. Although the perturbations in neurulation caused by genetic or environmental factors lead to neural tube defects (NTDs), the most common congenital malformation and the precise molecular pathological cascades mediating NTDs are not well understood. Recently, we have developed human spinal cord organoids (hSCOs) that recapitulate some aspects of human neurulation and observed that valproic acid (VPA) could cause neurulation defects in an organoid model. In this study, we identified and verified the significant changes in cell–cell junctional genes/proteins in VPA-treated organoids using transcriptomic and immunostaining analysis. Furthermore, VPA-treated mouse embryos exhibited impaired gene expression and NTD phenotypes, similar to those observed in the hSCO model. Collectively, our data demonstrate that hSCOs provide a valuable biological resource for dissecting the molecular pathways underlying the currently unknown human neurulation process using destructive biological analysis tools.
Figure
Reference
-
References
1. Schoenwolf GC, Smith JL. 1990; Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 109:243–270. DOI: 10.1242/dev.109.2.243. PMID: 2205465.
Article2. Copp AJ, Greene ND, Murdoch JN. 2003; The genetic basis of mammalian neurulation. Nat Rev Genet. 4:784–793. DOI: 10.1038/nrg1181. PMID: 13679871.
Article3. Colas JF, Schoenwolf GC. 2001; Towards a cellular and molecular understanding of neurulation. Dev Dyn. 221:117–145. DOI: 10.1002/dvdy.1144. PMID: 11376482.
Article4. Copp AJ, Stanier P, Greene ND. 2013; Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 12:799–810. DOI: 10.1016/S1474-4422(13)70110-8. PMID: 23790957. PMCID: PMC4023229.
Article5. Avagliano L, Massa V, George TM, Qureshy S, Bulfamante GP, Finnell RH. 2019; Overview on neural tube defects: from development to physical characteristics. Birth Defects Res. 111:1455–1467. DOI: 10.1002/bdr2.1380. PMID: 30421543. PMCID: PMC6511489.6. Harris MJ, Juriloff DM. 2010; An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol. 88:653–669. DOI: 10.1002/bdra.20676. PMID: 20740593.
Article7. Finnell RH, Caiaffa CD, Kim SE, et al. 2021; Gene environment interactions in the etiology of neural tube defects. Front Genet. 12:659612. DOI: 10.3389/fgene.2021.659612. PMID: 34040637. PMCID: PMC8143787. PMID: c93c33257ec34a14be44dd25ec610947.
Article8. Greene ND, Stanier P, Copp AJ. 2009; Genetics of human neural tube defects. Hum Mol Genet. 18(R2):R113–R129. DOI: 10.1093/hmg/ddp347. PMID: 19808787. PMCID: PMC2758708.
Article9. Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. 2015; Spina bifida. Nat Rev Dis Primers. 1:15007. DOI: 10.1038/nrdp.2015.7. PMID: 27189655. PMCID: PMC4898641.
Article10. Wang L, Li Z, Jin L, et al. 2014; Indoor air pollution and neural tube defects: effect modification by maternal genes. Epide-miology. 25:658–665. DOI: 10.1097/EDE.0000000000000129. PMID: 25051309.11. Shaw GM, Todoroff K, Velie EM, Lammer EJ. 1998; Maternal illness, including fever and medication use as risk factors for neural tube defects. Teratology. 57:1–7. DOI: 10.1002/(SICI)1096-9926(199801)57:1<1::AID-TERA1>3.0.CO;2-6. PMID: 9516745.
Article12. Suarez L, Felkner M, Brender JD, Canfield M, Hendricks K. 2008; Maternal exposures to cigarette smoke, alcohol, and street drugs and neural tube defect occurrence in offspring. Matern Child Health J. 12:394–401. DOI: 10.1007/s10995-007-0251-y. PMID: 17641961.
Article13. Lee JH, Shin H, Shaker MR, et al. 2022; Production of human spinal-cord organoids recapitulating neural-tube morpho-genesis. Nat Biomed Eng. 6:435–448. DOI: 10.1038/s41551-022-00868-4. PMID: 35347276.
Article14. Wegner C, Drews E, Nau H. 1990; Zinc concentrations in mouse embryo and maternal plasma. Effect of valproic acid and nonteratogenic metabolite. Biol Trace Elem Res. 25:211–217. DOI: 10.1007/BF02990416. PMID: 1698419.
Article15. Kim JY, Shaker MR, Lee JH, Lee B, Kim H, Sun W. 2017; Identification of molecular markers distinguishing adult neural stem cells in the subventricular and subcallosal zones. Anim Cells Syst (Seoul). 21:152–159. DOI: 10.1080/19768354.2017.1324522. PMID: 30460064. PMCID: PMC6138335.
Article16. Warde-Farley D, Donaldson SL, Comes O, et al. 2010; The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38:W214–W220. DOI: 10.1093/nar/gkq537. PMID: 20576703. PMCID: PMC2896186.
Article17. Ehlers K, Stürje H, Merker HJ, Nau H. 1992; Valproic acid-induced spina bifida: a mouse model. Teratology. 45:145–154. DOI: 10.1002/tera.1420450208. PMID: 1377411.
Article18. Hughes A, Greene NDE, Copp AJ, Galea GL. 2018; Valproic acid disrupts the biomechanics of late spinal neural tube closure in mouse embryos. Mech Dev. 149:20–26. DOI: 10.1016/j.mod.2017.12.001. PMID: 29225143. PMCID: PMC5846844.
Article19. Koo B, Choi B, Park H, Yoon KJ. 2019; Past, present, and future of brain organoid technology. Mol Cells. 42:617–627. DOI: 10.14348/molcells.2019.0162. PMID: 31564073. PMCID: PMC6776157.20. Jang H, Kim SH, Koh Y, Yoon KJ. 2022; Engineering brain organoids: toward mature neural circuitry with an intact cytoarchitecture. Int J Stem Cells. 15:41–59. DOI: 10.15283/ijsc22004. PMID: 35220291. PMCID: PMC8889333.
Article21. Lee JH, Sun W. 2022; Neural organoids, a versatile model for neuroscience. Mol Cells. 45:53–64. DOI: 10.14348/molcells.2022.2019. PMID: 35236780. PMCID: PMC8907004.
Article22. Susaimanickam PJ, Kiral FR, Park IH. 2022; Region specific brain organoids to study neurodevelopmental disorders. Int J Stem Cells. 15:26–40. DOI: 10.15283/ijsc22006. PMID: 35220290. PMCID: PMC8889336.
Article23. Haremaki T, Metzger JJ, Rito T, Ozair MZ, Etoc F, Brivanlou AH. 2019; Self-organizing neuruloids model develop-mental aspects of Huntington's disease in the ectodermal compartment. Nat Biotechnol. 37:1198–1208. DOI: 10.1038/s41587-019-0237-5. PMID: 31501559.
Article24. Karzbrun E, Khankhel AH, Megale HC, et al. 2021; Human neural tube morphogenesis in vitro by geometric constraints. Nature. 599:268–272. DOI: 10.1038/s41586-021-04026-9. PMID: 34707290. PMCID: PMC8828633.
Article25. Sahni G, Chang SY, Meng JTC, et al. 2021; A micropatterned human-specific neuroepithelial tissue for modeling gene and drug-induced neurodevelopmental defects. Adv Sci (Weinh). 8:2001100. DOI: 10.1002/advs.202001100. PMID: 33717833. PMCID: PMC7927627. PMID: 6bd0687b0fd64a559eddde24ea136a8c.
Article26. Knight GT, Lundin BF, Iyer N, et al. 2018; Engineering induction of singular neural rosette emergence within hPSC-derived tissues. Elife. 7:e37549. DOI: 10.7554/eLife.37549. PMID: 30371350. PMCID: PMC6205811. PMID: 1fa2e3a854e64c92958b13c035acd8cd.
Article27. Zhang XZ, Huo HQ, Zhu YQ, et al. 2022; Folic acid rescues valproic acid-induced morphogenesis inhibition in neural rosettes derived from human pluripotent stem cells. Front Cell Neurosci. 16:888152. DOI: 10.3389/fncel.2022.888152. PMID: 35651759. PMCID: PMC9148965. PMID: 51efcc5ad61242039f85a77c5902f5a1.
Article28. Ranga A, Girgin M, Meinhardt A, et al. 2016; Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci U S A. 113:E6831–E6839. DOI: 10.1073/pnas.1603529113. PMID: 27742791. PMCID: PMC5098636.
Article29. Zheng Y, Xue X, Resto-Irizarry AM, et al. 2019; Dorsal-ventral patterned neural cyst from human pluripotent stem cells in a neurogenic niche. Sci Adv. 5:eaax5933. DOI: 10.1126/sciadv.aax5933. PMID: 31844664. PMCID: PMC6905871.
Article30. Tung EW, Winn LM. 2011; Valproic acid increases formation of reactive oxygen species and induces apoptosis in postimplantation embryos: a role for oxidative stress in valproic acid-induced neural tube defects. Mol Pharmacol. 80:979–987. DOI: 10.1124/mol.111.072314. PMID: 21868484.
Article31. Wegner C, Nau H. 1992; Alteration of embryonic folate metabolism by valproic acid during organogenesis: implications for mechanism of teratogenesis. Neurology. 42(4 Suppl 5):17–24. PMID: 1574172.32. Lloyd KA. 2013; A scientific review: mechanisms of valproate-mediated teratogenesis. Bioscience Horiz. 6:hzt003. DOI: 10.1093/biohorizons/hzt003.
Article33. Li Z, Ge W, Li Y, Zhang Y, Zhao X, Hu J. 2021; Valproic acid enhance reprogramming of bactrian camel cells through promoting the expression of endogenous gene c-Myc and the process of angiogenesis. Int J Stem Cells. 14:191–202. DOI: 10.15283/ijsc20213. PMID: 33632993. PMCID: PMC8138656.
Article34. Lee Y, Kim D, Lee CJ. 2020; Suppressive effects of valproic acid on caudal fin regeneration in adult zebrafish. Anim Cells Syst (Seoul). 24:349–358. DOI: 10.1080/19768354.2020.1860126. PMID: 33456719. PMCID: PMC7782361. PMID: 513d8aa727c6419f96134d02a58aa432.
Article35. Elmazar MM, Nau H. 1992; Methotrexate increases valproic acid-induced developmental toxicity, in particular neural tube defects in mice. Teratog Carcinog Mutagen. 12:203–210. DOI: 10.1002/tcm.1770120503. PMID: 1363493.
Article36. Roy M, Leclerc D, Wu Q, Gupta S, Kruger WD, Rozen R. 2008; Valproic acid increases expression of methylenetetrahydrofolate reductase (MTHFR) and induces lower terato-genicity in MTHFR deficiency. J Cell Biochem. 105:467–476. DOI: 10.1002/jcb.21847. PMID: 18615588. PMCID: PMC2574752.
Article37. Sahakyan V, Pozzo E, Duelen R, Deprest J, Sampaolesi M. 2017; Methotrexate and valproic acid affect early neurogenesis of human amniotic fluid stem cells from myelomeningocele. Stem Cells Int. 2017:6101609. DOI: 10.1155/2017/6101609. PMID: 29056972. PMCID: PMC5615990. PMID: 42576ed90de6411e992b33d7e3206aca.
Article38. Muhsen M, Youngs J, Riu A, et al. 2021; Folic acid supplementation rescues valproic acid-induced developmental neurotoxicity and behavioral alterations in zebrafish embryos. Epilepsia. 62:1689–1700. DOI: 10.1111/epi.16915. PMID: 33997963.
Article39. Cao R, Xie J, Zhang L. 2022; Abnormal methylation caused by folic acid deficiency in neural tube defects. Open Life Sci. 17:1679–1688. DOI: 10.1515/biol-2022-0504. PMID: 36589786. PMCID: PMC9784971. PMID: ce9b40f59ef3411bb8bf14b413d91cef.
Article40. Bjorklund NK, Gordon R. 2006; A hypothesis linking low folate intake to neural tube defects due to failure of post-translation methylations of the cytoskeleton. Int J Dev Biol. 50:135–141. DOI: 10.1387/ijdb.052102nb. PMID: 16479482.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Differential Developmental Neurotoxicity of Valproic Acid on Anterior and Posterior Neural Induction of Human Pluripotent Stem Cells
- Combined Method of Neuronal Cell-Inducible Vector and Valproic Acid for Enhanced Gene Expression under Hypoxic Conditions
- Spinal Dysraphism and Tethered Cord Syndrome
- Diastematomyelia associated with clubfoot: A Case Report
- Effects of Valproic Acid on the Survival of Human Tennon's Capsule Fibroblasts