J Korean Neurosurg Soc.
2020 Mar;63(2):188-201. 10.3340/jkns.2019.0131.
Single Centre Experience on Decision Making for Mechanical Thrombectomy Based on Single-Phase CT Angiography by Including NCCT and Maximum Intensity Projection Images – A Comparison with Magnetic Resonance Imaging after Non-Contrast CT
- Affiliations
-
- 1Department of Neurosurgery, Dong Kang Medical Center, Ulsan, Korea
- 2Department of Radiology, Dong Kang Medical Center, Ulsan, Korea
- KMID: 2501705
- DOI: http://doi.org/10.3340/jkns.2019.0131
Abstract
Objective
: The purpose of this study was to suggest that computed tomography angiography (CTA) is valuable as the only preliminary examination for mechanical thrombectomy (MT). MT after single examination of CTA including non-contrast computed tomography (NCCT) and maximum intensity projection (MIP) improves door-to-puncture time as well as results in favorable outcomes.
Methods
: A total of 157 patients who underwent MT at Dong Kang Medical Center from April 2015 to March 2019 were divided into two groups based on the examination performed prior to MT : CTA group who underwent CTA with NCCT and MIP, and NCCT+magnetic resonance image (MRi) group who underwent MRI including perfusion images after NCCT. In the two groups, time to CTA imaging or NCCT+MRi imaging after symptom onset, and time to arterial puncture and reperfusion were characterized as time-related outcomes. The evaluation of vascular recanalization after MT was defined as a modified thrombolysis in cerebral infarction (mTICI) scale. National Institutes of Health Stroke Scale (NIHSS) was assessed at the time of the visit to the emergency room and modified Rankin Scale (mRS) was assessed after 90 days.
Results
: Typically, there were 34 patients in the CTA group and 33 patients in the NCCT+MRi group. A significantly shorter delay for door-to-puncture time was observed (mean, 86±22.1 vs. 176±47.5 minutes; p<0.01). Also, a significantly shorter door-to-imege time in the CTA group was observed (mean, 13±6.8 vs. 93±30.8 minutes; p<0.01). Moreover, a significantly shorter onset-to-puncture time was observed (mean, 195±128.0 vs. 314±157.6 minutes; p<0.01). Reperfusion result of mTICI ≥2b was 100% (34/34) in the CTA group and 94% (31/33) in the NCCT+MRi group, and mTICI 3 in 74% (25/34) in the CTA group and 73% (24/33) in the NCCT+MRi group. Favorable functional outcomes (mRS score ≤2 at 90 days) were 68% (23/34) in the CTA group and 60% (20/33) in the NCCT+MRi group.
Conclusion
: A single-phase CTA including NCCT and MIP images was performed as a single preliminary examination, which led to a reduction in the time of the procedure and resulted in good results of prognosis. Consequently, it is concluded that this method is of sufficient value as the only preliminary examination for decision making.
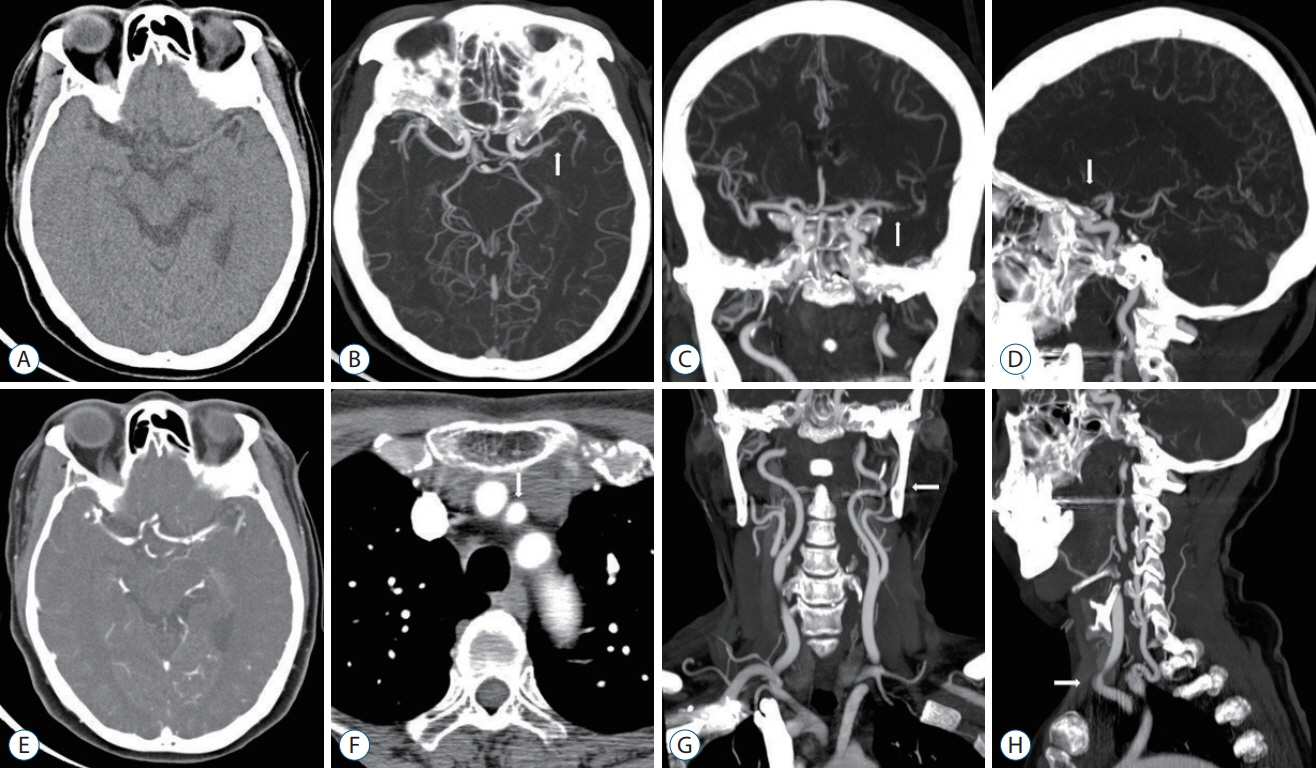
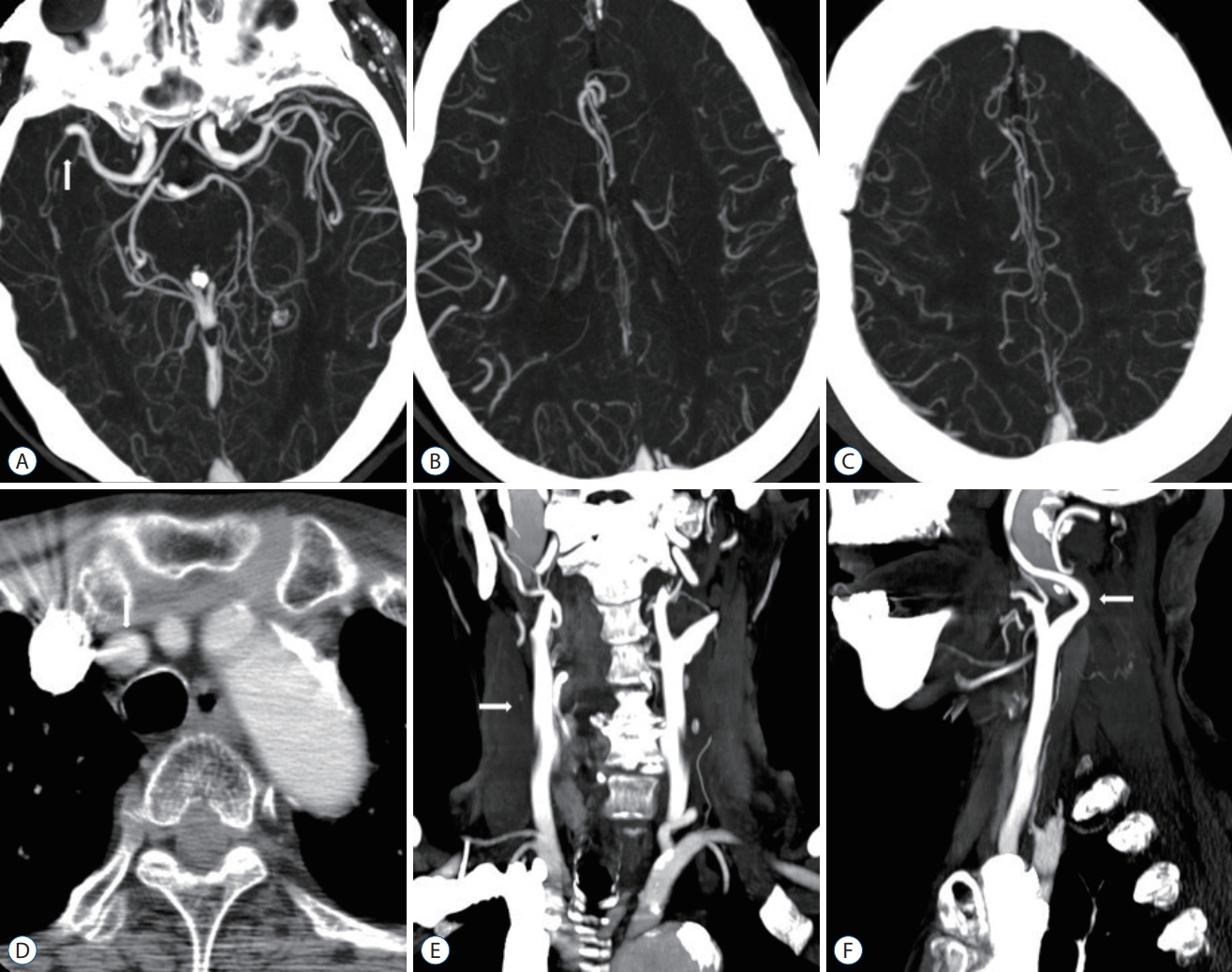
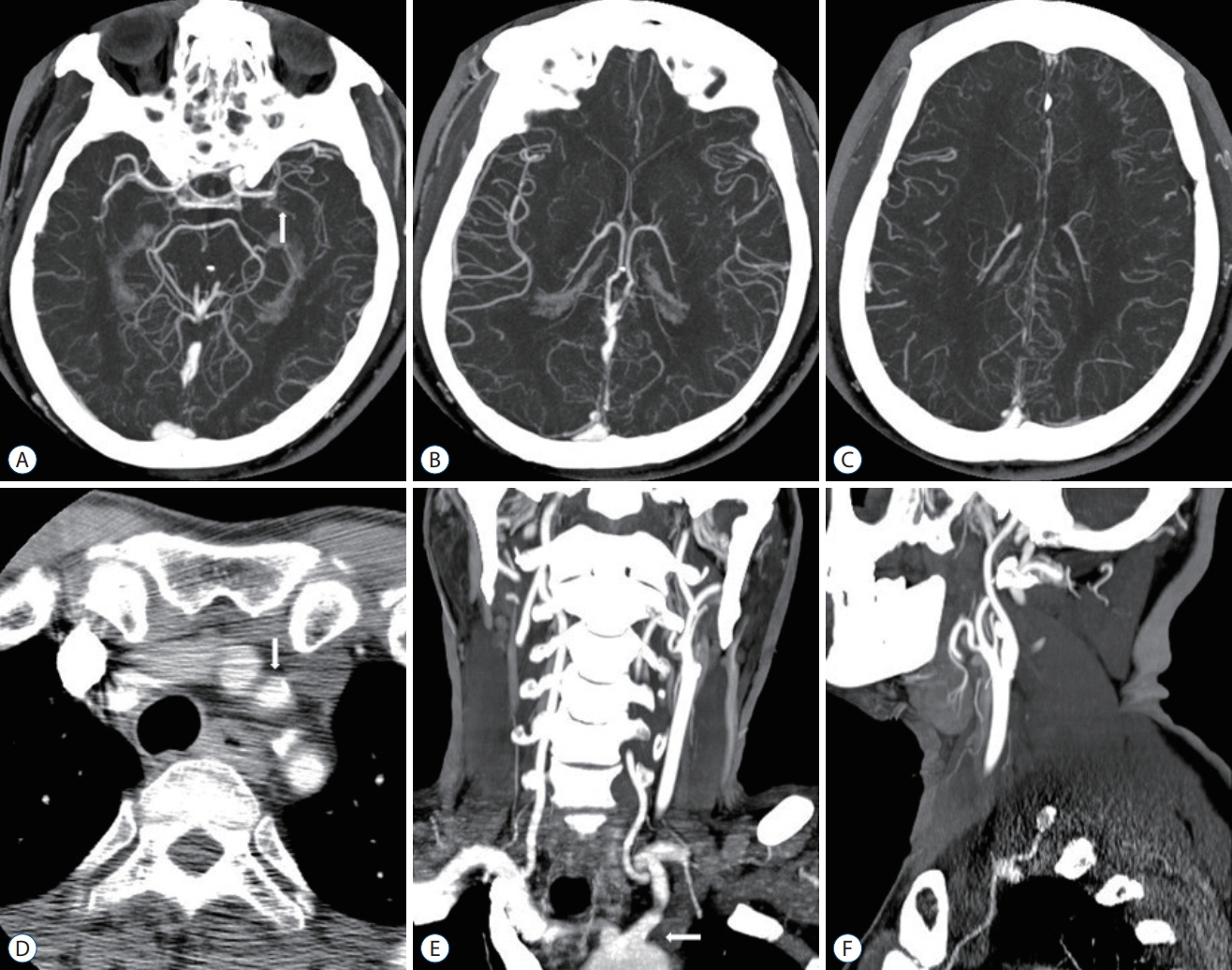
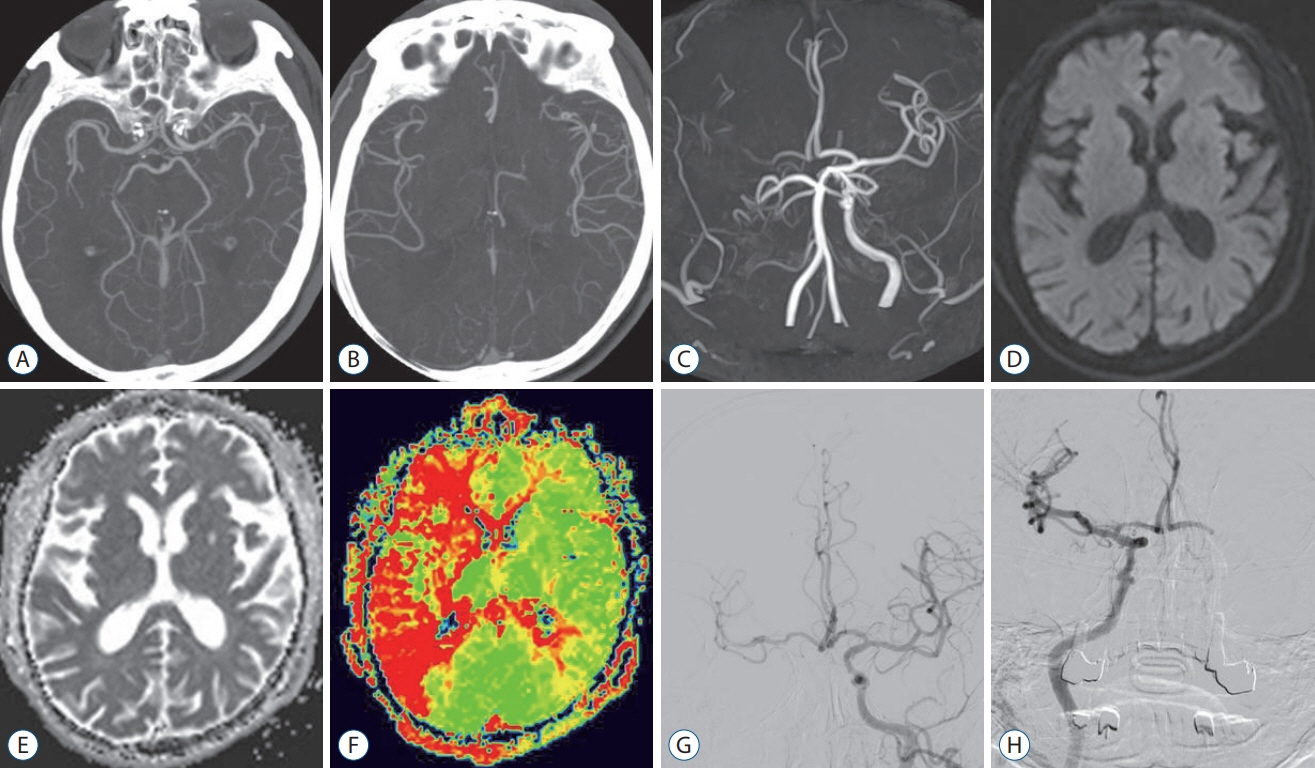
Figure
Reference
-
References
1. Aghaebrahim A, Streib C, Rangaraju S, Kenmuir CL, Giurgiutiu DV, Horev A, et al. Streamlining door to recanalization processes in endovascular stroke therapy. J Neurointerv Surg. 9:340–345. 2017.
Article2. Akins PT, Amar AP, Pakbaz RS, Fields JD, SWIFT Investigators. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices. AJNR Am J Neuroradiol. 35:524–528. 2014.
Article3. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 372:11–20. 2015.4. Brinjikji W, Demchuk AM, Murad MH, Rabinstein AA, McDonald RJ, McDonald JS, et al. Neurons over nephrons: systematic review and metaanalysis of contrast-induced nephropathy in patients with acute stroke. Stroke. 48:1862–1868. 2017.5. Broderick JP, Schroth G. What the SWIFT and TREVO II trials tell us about the role of endovascular therapy for acute stroke. Stroke. 44:1761–1764. 2013.
Article6. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 372:1009–1018. 2015.
Article7. Campbell BCV, Donnan GA, Mitchell PJ, Davis SM. Endovascular thrombectomy for stroke: current best practice and future goals. Stroke Vasc Neurol. 1:16–22. 2016.
Article8. Casaubon LK, Boulanger JM, Blacquiere D, Boucher S, Brown K, Goddard T, et al. Canadian stroke best practice recommendations: hyperacute stroke care guidelines, update 2015. Int J Stroke. 10:924–940. 2015.
Article9. Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 369:293–298. 2007.
Article10. Choi Y, Chung SB, Kim MS. Prevalence and anatomy of aberrant right subclavian artery evaluated by computed tomographic angiography at a single institusion in Korea. J Korean Neurosurg Soc. 62:175–182. 2019.
Article11. Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD, et al. CT/ CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke. 43:1013–1017. 2012.
Article12. Danière F, Lobotesis K, Machi P, Eker O, Mourand I, Riquelme C, et al. Patient selection for stroke endovascular therapy--DWI-ASPECTS thresholds should vary among age groups: insights from the RECOST study. AJNR Am J Neuroradiol. 36:32–39. 2015.13. de Weerd L, Luijckx GJ, Groenier KH, van der Meer K. Quality of life of elderly ischaemic stroke patients one year after thrombolytic therapy. A comparison between patients with and without thrombolytic therapy. BMC Neurol. 12:61. 2012.
Article14. Frölich AM, Wolff SL, Psychogios MN, Klotz E, Schramm R, Wasser K, et al. Time-resolved assessment of collateral flow using 4D CT angiography in large-vessel occlusion stroke. Eur Radiol. 24:390–396. 2014.
Article15. García-Tornel A, Carvalho V, Boned S, Flores A, Rodríguez-Luna D, Pagola J, et al. Improving the evaluation of collateral circulation by multiphase computed tomography angiography in acute stroke patients treated with endovascular reperfusion therapies. Interv Neurol. 5:209–217. 2016.
Article16. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 372:1019–1030. 2015.
Article17. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 359:1317–1329. 2008.
Article18. IMS II Trial Investigators. The interventional management of stroke (IMS) II study. Stroke. 38:2127–2135. 2007.
Article19. Jankowitz B, Aghaebrahim A, Zirra A, Spataru O, Zaidi S, Jumaa M, et al. Manual aspiration thrombectomy: adjunctive endovascular recanalization technique in acute stroke interventions. Stroke. 43:1408–1411. 2012.20. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 44:870–947. 2013.
Article21. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 372:2296–2306. 2015.
Article22. Kang DH, Hwang YH, Kim YS, Park J, Kwon O, Jung C. Direct thrombus retrieval using the reperfusion catheter of the penumbra system: forcedsuction thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol. 32:283–287. 2011.
Article23. Kang DW, Chalela JA, Dunn W, Warach S, NIH-Suburban Stroke Center Investigators. MRI screening before standard tissue plasminogen activator therapy is feasible and safe. Stroke. 36:1939–1943. 2005.
Article24. Kim BJ, Kang HG, Kim HJ, Ahn SH, Kim NY, Warach S, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Storke. 16:131–145. 2014.
Article25. Kim DH, Kim B, Jung C, Nam HS, Lee JS, Kim JW, et al. Consensus statements by Korean Society of Interventional Neuroradiology and Korean Stroke Society: hyperacute endovascular treatment workflow to reduce door-to-reperfusion time. J Korean Med Sci. 33:e143. 2018.
Article26. Kim TK, Rhim JK, Lee CJ, Oh SH, Chung BS. The limitations of thrombectomy with Solitaire™ AB as first-line treatment in acute ischemic stroke: a single center experience. J Cerebrovasc Endovasc Neurosurg. 14:203–209. 2012.
Article27. Lee JS, Demchuk AM. Choosing a hyperacute stroke imaging protocol for proper patient selection and time efficient endovascular treatment: lessons from recent trials. J Stroke. 17:221–228. 2015.
Article28. Lee KY, Han SW, Kim SH, Nam HS, Ahn SW, Kim DJ, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke. 38:192–193. 2007.
Article29. Lev MH, Farkas J, Rodriguez VR, Schwamm LH, Hunter GJ, Putman CM, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr. 25:520–528. 2001.
Article30. Mehta BP, Leslie-Mazwi TM, Chandra RV, Bell DL, Sun CH, Hirsch JA, et al. Reducing door-to-puncture times for intra-arterial stroke therapy: a pilot quality improvement project. J Am Heart Assoc. 3:e000963. 2014.
Article31. Menon BK, Almekhlafi MA, Pereira VM, Gralla J, Bonafe A, Chapot R, et al. Optimal workflow and process-based performance measures for endovascular therapy in acute ischemic stroke: analysis of the Solitaire FR thrombectomy for acute revascularization study. Stroke. 45:2024–2029. 2014.
Article32. Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 275:510–520. 2015.
Article33. Menon BK, Goyal M. Endovascular therapy in acute ischemic stroke: where we are, the challenges we face and what the future holds. Expert Rev Cardiovasc Ther. 9:473–484. 2011.
Article34. Menon BK, O’Brien B, Bivard A, Spratt NJ, Demchuk AM, Miteff F, et al. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. J Cereb Blood Flow Metab. 33:365–371. 2013.
Article35. Merino JG, Warach S. Imaging of acute stroke. Nat Rev Neurol. 6:560–571. 2010.
Article36. Molina CA, Chamorro A, Rovira À, de Miquel A, Serena J, Roman LS, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eighthours of symptom onset. Int J Stroke. 10:619–626. 2015.
Article37. Nogueira RG, Lutseg HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 380:1231–1240. 2012.
Article38. Pereira VM, Gralla J, Davalos A, Bonafé A, Castaño C, Chapot R, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke. 44:2802–2807. 2013.
Article39. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 46:3020–3035. 2015.
Article40. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a metaanalysis. Stroke. 38:967–973. 2007.
Article41. Ribo M, Molina CA, Cobo E, Cerdà N, Tomasello A, Quesada H, et al. Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke. 47:999–1004. 2016.
Article42. Riedel CH, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke. 43:2319–2323. 2012.
Article43. Roth C, Papanagiotou P, Behnke S, Walter S, Haass A, Becker C, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke. 41:2559–2567. 2010.
Article44. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 372:2285–2295. 2015.
Article45. Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 316:1279–1288. 2016.
Article46. Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, noninferiority trial. Lancet. 380:1241–1249. 2012.
Article47. Sharma J, Nanda A, Jung RS, Mehta S, Pooria J, Hsu DP. Risk of contrast-induced nephropathy in patients undergoing endovascular treatment of acute ischemic stroke. J Neurointerv Surg. 5:543–545. 2013.
Article48. Singer OC, Sitzer M, du Mesnil de Rochemont R, Neumann-Haefelin T. Practical limitations of acute stroke MRI due to patient-related problems. Neurology. 62:1848–1849. 2004.
Article49. Turk AS, Spiotta A, Frei D, Mocco J, Baxter B, Fiorella D, et al. Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg. 6:231–237. 2014.
Article50. Yoo SH, Kwon SU, Lee DH, Kim SJ, Kim JS, Kang DW. Comparison between MRI screening and CT-plus-MRI screening for thrombolysis within 3 h of ischemic stroke. J Neurol Sci. 294:119–123. 2010.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Artifacts in MR Angiography of the Intracranial Vessels Using the 3D TOF and 3D PC Techniques
- MRCP Using Breath-hold HASTE Sequence: Comparison of Maximum Intensity Projection Image with Single Slice Acquisition Image
- Abbreviated Breast Magnetic Resonance Imaging: Background, Evidence From Studies, and Future Considerations
- Image Quality of the 3 Dimensional Phase-Contrast Technique in an Intracranial Magnetic Resonance Angiography with Artifacts Caused by Orthodontic Devices: A Comparison with 3 Dimensional Time-of-Flight Technique
- Three-dimensional CT angiography of the canine hepatic vasculature