Recent updates on the basic mechanisms and pathogenesis of inflammatory bowel diseases in experimental animal models
- Affiliations
-
- 1Department of Immunology, Kurume University School of Medicine, Kurume, Japan
- 2Department of Molecular Microbiology and Immunology, Brown University Warren Alpert Medical School, Providence, RI, USA
- 3Crohn’s & Colitis Society of Singapore, Singapore
- KMID: 2501381
- DOI: http://doi.org/10.5217/ir.2019.09154
Abstract
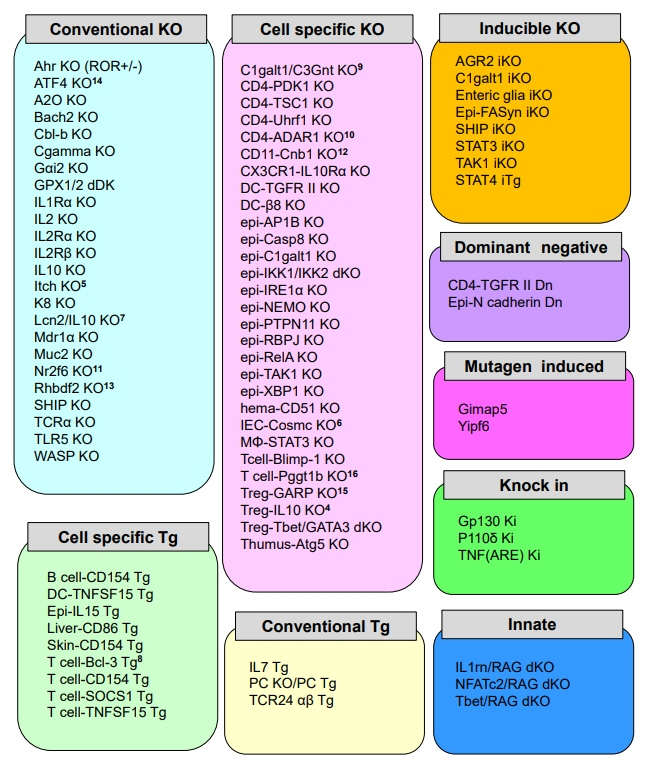
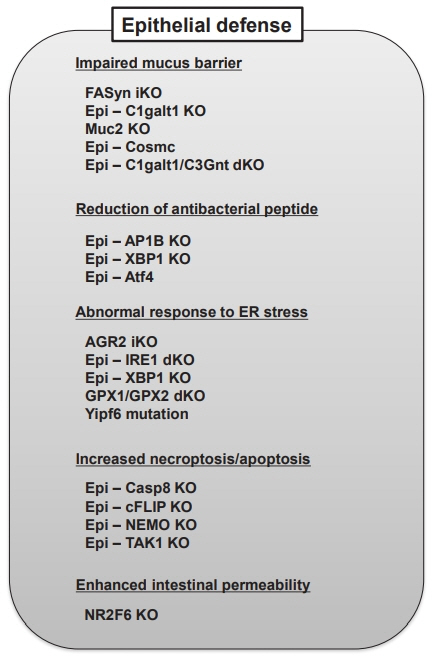
- The specific pathogenesis underlining inflammatory bowel disease (IBD) is very complicated, and it is further more difficult to clearly explain the pathophysiology of 2 major forms of IBD, Crohn’s disease (CD) and ulcerative colitis (UC), and both disorders affect individuals throughout life. Despite every extensive effort, the interplay among genetic factors, immunological factors, environmental factors and intestinal microbes is still completely unrevealed. Animal models are indispensable to find out mechanistic details that will facilitate better preclinical setting to target specific components involved in the pathogenesis of IBD. Based on many recent reports, dysbiosis of the commensal microbiota is implicated in the pathogenesis of several diseases, not only IBD but also colon cancer, obesity, psoriasis as well as allergic disorders, in both human and animal models. Advanced technologies including cell-specific and inducible knockout systems, which are recently employed to mouse IBD models, have further enhanced the ability of developing new therapeutic strategies for IBD. Furthermore, data from these mouse models highlight the critical involvement of dysregulated immune responses and impaired colonic epithelial defense system in the pathogenesis of IBD. In this review, we will explain from the history of animal models of IBD to the recent reports of the latest compounds, therapeutic strategies, and approaches tested on IBD animal models.
Keyword
Figure
Cited by 6 articles
-
Succinate-treated macrophages attenuate dextran sodium sulfate colitis in mice
I Seul Park, Mijeong Son, Hyun Woo Ma, Jihyung Kim, Da Hye Kim, Seung Won Kim, Jae Hee Cheon
Intest Res. 2021;19(3):349-353. doi: 10.5217/ir.2020.00075.Anti-inflammatory properties of
Escherichia coli Nissle 1917 in a murine colitis model
Jihye Park, Da Hye Kim, Soochan Kim, Hyun Woo Ma, I Seul Park, Mijeong Son, Ji Hyung Kim, Yoojin Shin, Seung Won Kim, Jae Hee Cheon
Intest Res. 2021;19(4):478-481. doi: 10.5217/ir.2021.00121.Update on the epidemiology of inflammatory bowel disease in Asia: where are we now?
Sang Hyoung Park
Intest Res. 2022;20(2):159-164. doi: 10.5217/ir.2021.00115.Compositional changes in fecal microbiota associated with clinical phenotypes and prognosis in Korean patients with inflammatory bowel disease
Seung Yong Shin, Young Kim, Won-Seok Kim, Jung Min Moon, Kang-Moon Lee, Sung-Ae Jung, Hyesook Park, Eun Young Huh, Byung Chang Kim, Soo Chan Lee, Chang Hwan Choi
Intest Res. 2023;21(1):148-160. doi: 10.5217/ir.2021.00168.Korean clinical practice guidelines on biologics for moderate to severe Crohn’s disease
Seong-Joon Koh, Sung Noh Hong, Soo-Kyung Park, Byong Duk Ye, Kyeong Ok Kim, Jeong Eun Shin, Yong Sik Yoon, Hong Sub Lee, Sung Hoon Jung, Miyoung Choi, Soo-Young Na, Chang Hwan Choi, Joo Sung Kim
Intest Res. 2023;21(1):43-60. doi: 10.5217/ir.2022.00029.Regional variations in the prevalence of primary sclerosing cholangitis associated with inflammatory bowel disease
Kwang Woo Kim, Hyoun Woo Kang
Intest Res. 2023;21(4):413-414. doi: 10.5217/ir.2023.00133.
Reference
-
1. Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993; 75:263–274.
Article2. Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993; 75:253–261.
Article3. Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993; 75:274–282.
Article4. Mizoguchi A, Takeuchi T, Himuro H, Okada T, Mizoguchi E. Genetically engineered mouse models for studying inflammatory bowel disease. J Pathol. 2016; 238:205–219.
Article5. Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther. 2013; 7:1341–1357.6. Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019; 20:970–979.
Article7. DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016; 22:1137–1150.
Article8. Shanahan F. The colonic microbiota in health and disease. Curr Opin Gastroenterol. 2013; 29:49–54.
Article9. Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013; 62:1505–1510.
Article10. Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci. 2012; 105:263–320.
Article11. Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011; 43:246–252.12. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009; 361:2066–2078.
Article13. Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010; 42:1118–1125.14. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011; 474:307–317.
Article15. Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009; 361:2033–2045.
Article16. Shouval DS, Biswas A, Goettel JA, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014; 40:706–719.
Article17. Mizoguchi E, Mizoguchi A, Bhan AK. Insights from recent advances in animal models of inflammatory bowel disease. In : D’Amato M, Rioux JD, editors. Molecular genetics of inflammatory bowel disease. New York: Springer;2013. p. 45–83.18. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007; 2:541–546.
Article19. Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007; 13:5581–5593.
Article20. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990; 98:694–702.
Article21. Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989; 96:795–803.
Article22. Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995; 182:1281–1290.
Article23. Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009; 15:341–352.
Article24. Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998; 188:1929–1939.
Article25. MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978; 17:135–150.
Article26. Dieleman LA, Elson CO, Tennyson GS, Beagley KW. Kinetics of cytokine expression during healing of acute colitis in mice. Am J Physiol. 1996; Jul. (1 Pt 1):G130–G136.
Article27. Yamada T, Deitch E, Specian RD, Perry MA, Sartor RB, Grisham MB. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993; 17:641–662.
Article28. Lee WT, Yin XM, Vitetta ES. Functional and ontogenetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J Immunol. 1990; 144:3288–3295.
Article29. Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993; 5:1461–1471.
Article30. Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice: disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993; 178:237–244.
Article31. Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G135–G146.
Article32. Sundberg JP, Elson CO, Bedigian H, Birkenmeier EH. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 1994; 107:1726–1735.
Article33. Bristol IJ, Farmer MA, Cong Y, et al. Heritable susceptibility for colitis in mice induced by IL-10 deficiency. Inflamm Bowel Dis. 2000; 6:290–302.
Article34. Kosiewicz MM, Nast CC, Krishnan A, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J Clin Invest. 2001; 107:695–702.
Article35. Bamias G, Martin C, Mishina M, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005; 128:654–666.
Article36. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001; 411:599–603.
Article37. Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006; 314:1461–1463.
Article38. Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009; 58:1152–1167.
Article39. Mizoguchi A, Mizoguchi E. Animal models of IBD: linkage to human disease. Curr Opin Pharmacol. 2010; 10:578–587.
Article40. Kuballa P, Huett A, Rioux JD, Daly MJ, Xavier RJ. Impaired autophagy of an intracellular pathogen induced by a Crohn’s disease associated ATG16L1 variant. PLoS One. 2008; 3:e3391.
Article41. Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008; 456:264–268.
Article42. Hitotsumatsu O, Ahmad RC, Tavares R, et al. The ubiquitinediting enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008; 28:381–390.
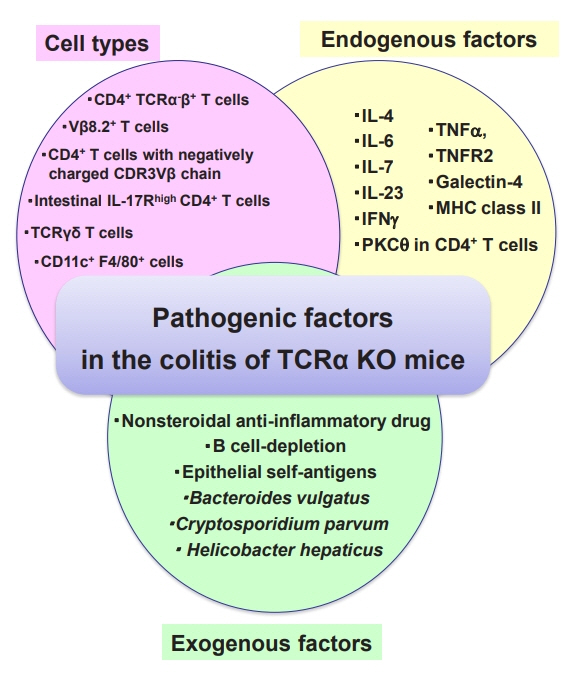
Article43. Mizoguchi A, Mizoguchi E, Chiba C, et al. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996; 183:847–856.
Article44. Mizoguchi A, Mizoguchi E, Chiba C, Bhan AK. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med. 1996; 184:707–715.
Article45. Bhan AK, Mizoguchi E, Smith RN, Mizoguchi A. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol Rev. 1999; 169:195–207.
Article46. Iijima H, Takahashi I, Kishi D, et al. Alteration of interleukin 4 production results in the inhibition of T helper type 2 cell-dominated inflammatory bowel disease in T cell receptor alpha chain-deficient mice. J Exp Med. 1999; 190:607–615.47. Mizoguchi A, Mizoguchi E, Saubermann LJ, Higaki K, Blumberg RS, Bhan AK. Limited CD4 T-cell diversity associated with colitis in T-cell receptor alpha mutant mice requires a T helper 2 environment. Gastroenterology. 2000; 119:983–995.
Article48. Morgan NV, Goddard S, Cardno TS, et al. Mutation in the TCRα subunit constant gene (TRAC) leads to a human immunodeficiency disorder characterized by a lack of TCRαβ+ T cells. J Clin Invest. 2011; 121:695–702.
Article49. Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997; 150:91–97.50. Chin EY, Dangler CA, Fox JG, Schauer DB. Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor alphabeta mutant mice. Comp Med. 2000; 50:586–594.51. Burich A, Hershberg R, Waggie K, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001; 281–G764G778.52. Gaskins HR, Vondrak-Juergens GL, McCracken BA, Woolsey JH. Specific-pathogen-free conditions enhance inflammatory bowel disease in T-cell receptor knockout, but not C3H/ HeJBir mice. Lab Anim Sci. 1997; 47:650–655.53. Shimomura Y, Mizoguchi E, Sugimoto K, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008; 20:729737.
Article54. Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006; 176:705–710.
Article55. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002; 16:219–230.
Article56. Sugimoto K, Ogawa A, Shimomura Y, Nagahama K, Mizoguchi A, Bhan AK. Inducible IL-12-producing B cells regulate Th2-mediated intestinal inflammation. Gastroenterology. 2007; 133:124–136.
Article57. Leiper K, Martin K, Ellis A, et al. Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut. 2011; 60:1520–1526.
Article58. Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007; 13:1365–1368.
Article59. Ardelean DS, Gonska T, Wires S, et al. Severe ulcerative colitis after rituximab therapy. Pediatrics. 2010; 126:e243–e246.
Article60. Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012; 156:350–359.
Article61. Matharu KS, Mizoguchi E, Cotoner CA, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology. 2009; 137:1380–1390.
Article62. Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998; 66:5224–5231.
Article63. Hoentjen F, Harmsen HJ, Braat H, et al. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut. 2003; 52:1721–1727.
Article64. Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998; 161:3143–3149.
Article65. Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006; 296:1731–1732.
Article66. Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014; 6:114–118.67. Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013; 91:207–213.
Article68. Beattie DT, Pulido-Rios MT, Shen F, et al. Intestinally-restricted Janus kinase inhibition: a potential approach to maximize the therapeutic index in inflammatory bowel disease therapy. J Inflamm (Lond). 2017; 14:28.
Article69. Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014; 12:1485–1493.70. Menet CJ, Fletcher SR, van Lommen G, et al. Triazolopyridines as selective JAK1 inhibitors: from hit identification to GLPG0634. J Med Chem. 2014; 57:9323–9342.
Article71. Namour F, Diderichsen PM, Cox E, et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of Filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin Pharmacokinet. 2015; 54:859–874.
Article72. Parmentier JM, Voss J, Graff C, et al. in vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT494). BMC Rheumatol. 2018; 2:23.73. Nielsen OH, Li Y, Johansson-Lindbom B, Coskun M. Sphingosine-1-phosphate signaling in inflammatory bowel disease. Trends Mol Med. 2017; 23:362–374.
Article74. Karuppuchamy T, Behrens EH, González-Cabrera P, et al. Sphingosine-1-phosphate receptor-1 (S1P(1)) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 2017; 10:162–171.
Article75. Scott FL, Clemons B, Brooks J, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016; 173:1778–1792.
Article76. Peyrin-Biroulet L, Panés J, Chiorean M, et al. Histological remission and mucosal healing in a randomised, placebo-controlled, phase 2 study of etrasimod in patients with moderately to severely active ulcerative colitis. J Crohns Colitis. 2019; 13:S6.77. Sugiura T, Kageyama S, Andou A, et al. Oral treatment with a novel small molecule alpha 4 integrin antagonist, AJM300, prevents the development of experimental colitis in mice. J Crohns Colitis. 2013; 7:e533–e542.
Article78. Yoshimura N, Watanabe M, Motoya S, et al. Safety and efficacy of AJM300, an oral antagonist of α4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology. 2015; 149:1775–1783.
Article79. Wermers JD, McNamee EN, Wurbel MA, Jedlicka P, RiveraNieves J. The chemokine receptor CCR9 is required for the Tcell-mediated regulation of chronic ileitis in mice. Gastroenterology. 2011; 140:1526–1535.
Article80. Keshav S, Vaňásek T, Niv Y, et al. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS One. 2013; 8:e60094.
Article81. Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994; 1:553–562.
Article82. Olson AD, DelBuono EA, Bitar KN, Remick DG. Antiserum to tumor necrosis factor and failure to prevent murine colitis. J Pediatr Gastroenterol Nutr. 1995; 21:410–418.
Article83. Rennick DM, Fort MM, Davidson NJ. Studies with IL-10-/- mice: an overview. J Leukoc Biol. 1997; 61:389–396.
Article84. Berg DJ, Davidson N, Kühn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996; 98:1010–1020.
Article85. Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002; 17:629–638.
Article86. Hoving JC, Kirstein F, Nieuwenhuizen NE, et al. B cells that produce immunoglobulin E mediate colitis in BALB/c mice. Gastroenterology. 2012; 142:96–108.
Article87. Davies SC, Nguyen TM, Parker CE, MacDonald JK, Jairath V, Khanna R. Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2019; 12:CD012804.
Article88. Verstockt B, Ferrante M, Vermeire S, van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018; 53:585–590.89. Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000; 6:583–588.
Article90. Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000; 164:4878–4882.
Article91. Park SC, Jeen YT. Anti-integrin therapy for inflammatory bowel disease. World J Gastroenterol. 2018; 24:1868–1880.
Article92. Zundler S, Schillinger D, Fischer A, et al. Blockade of αEβ7 integrin suppresses accumulation of CD8(+) and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut. 2017; 66:1936–1948.
Article93. Khan I, Ullah N, Zha L, et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. 2019; 8–E126.
Article94. Burrello C, Giuffrè MR, Macandog AD, et al. Fecal microbiota transplantation controls murine chronic intestinal inflammation by modulating immune cell functions and gut microbiota composition. Cells. 2019; 8–E517.
Article95. Burrello C, Garavaglia F, Cribiù FM, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun. 2018; 9:5184.
Article96. McCoy KD, Geuking MB, Ronchi F. Gut microbiome standardization in control and experimental mice. Curr Protoc Immunol. 2017; 117:23. 1.1-23.1.13.
Article97. Koboziev I, Jones-Hall Y, Valentine JF, Webb CR, Furr KL, Grisham MB. Use of humanized mice to study the pathogenesis of autoimmune and inflammatory diseases. Inflamm Bowel Dis. 2015; 21:1652–1673.
Article98. Goettel JA, Gandhi R, Kenison JE, et al. AHR activation is protective against colitis driven by T cells in humanized mice. Cell Rep. 2016; 17:1318–1329.
Article99. Goettel JA, Kotlarz D, Emani R, et al. Low-dose interleukin-2 ameliorates colitis in a preclinical humanized mouse model. Cell Mol Gastroenterol Hepatol. 2019; 8:193–195.
Article100. Kozlowski C, Jeet S, Beyer J, et al. An entirely automated method to score DSS-induced colitis in mice by digital image analysis of pathology slides. Dis Model Mech. 2013; 6:855–865.
Article101. Romagnoni A, Jégou S, van Steen K, Wainrib G, Hugot JP; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC). Comparative performances of machine learning methods for classifying Crohn disease patients using genome-wide genotyping data. Sci Rep. 2019; 9:10351.102. Moran CJ, Klein C, Muise AM, Snapper SB. Very early-onset inflammatory bowel disease: gaining insight through focused discovery. Inflamm Bowel Dis. 2015; 21:1166–1175.103. Shin W, Kim HJ. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc Natl Acad Sci U S A. 2018; 115:E10539–E10547.
Article104. Xu P, Becker H, Elizalde M, Masclee A, Jonkers D. Intestinal organoid culture model is a valuable system to study epithelial barrier function in IBD. Gut. 2018; 67:1905–1906.
Article105. Sarvestani SK, Signs SA, Lefebvre V, et al. Cancer-predicting transcriptomic and epigenetic signatures revealed for ulcerative colitis in patient-derived epithelial organoids. Oncotarget. 2018; 9:28717–28730.
Article106. Yoo JH, Donowitz M. Intestinal enteroids/organoids: a novel platform for drug discovery in inflammatory bowel diseases. World J Gastroenterol. 2019; 25:4125–4147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Animal Models of Inflammatory Bowel Disease
- Pathogenesis of Inflammatory Bowel Diseases
- Animal Model for Inflammatory Bowel Disease
- Experimental Animal Models for Rheumatoid Arthritis: Methods and Applications
- Environmental and Microbial Factors in Inflammatory Bowel Disease Model Establishment: A Review Partly through Mendelian Randomization