Cancer Res Treat.
2020 Jan;52(1):128-138. 10.4143/crt.2019.119.
Identification of Significant Prognostic Tissue Markers Associated with Survival in Upper Urinary Tract Urothelial Carcinoma Patients Treated with Radical Nephroureterectomy: A Retrospective Immunohistochemical Analysis Using Tissue Microarray
- Affiliations
-
- 1Department of Urology, Urologic Cancer, Research Institute and Hospital of National Cancer Center, Goyang, Korea
- 2Department of Pathology, Hospital of National Cancer Center, Goyang, Korea
- 3Biostatistics Collaboration Unit, Research Institute of National Cancer Center, Goyang, Korea
- KMID: 2501208
- DOI: http://doi.org/10.4143/crt.2019.119
Abstract
- Purpose
The purpose of this study was to identify prognostic tissue markers for several survival outcomes after radical nephroureterectomy among patients with upper urinary tract urothelial carcinoma using tissue microarray and immunohistochemistry.
Materials and Methods
Retrospectively, data of 162 non-metastatic patients with upper urinary tract urothelial carcinoma after radical nephroureterectomy between 2004 and 2016 were reviewed to determine intravesical recurrence-free survival (IVRFS), disease-free survival (DFS), and overall survival (OS). The expression of 27 tissue markers on a tissue microarray of radical nephroureterectomy samples and prognostic values of clinicopathological parameters were evaluated using immunohistochemistry and Cox proportional hazard models after adjusting for significant prognostic clinicopathological variables. The expression of all tissue markers was categorized into a binary group with continuous H-scores (0-300).
Results
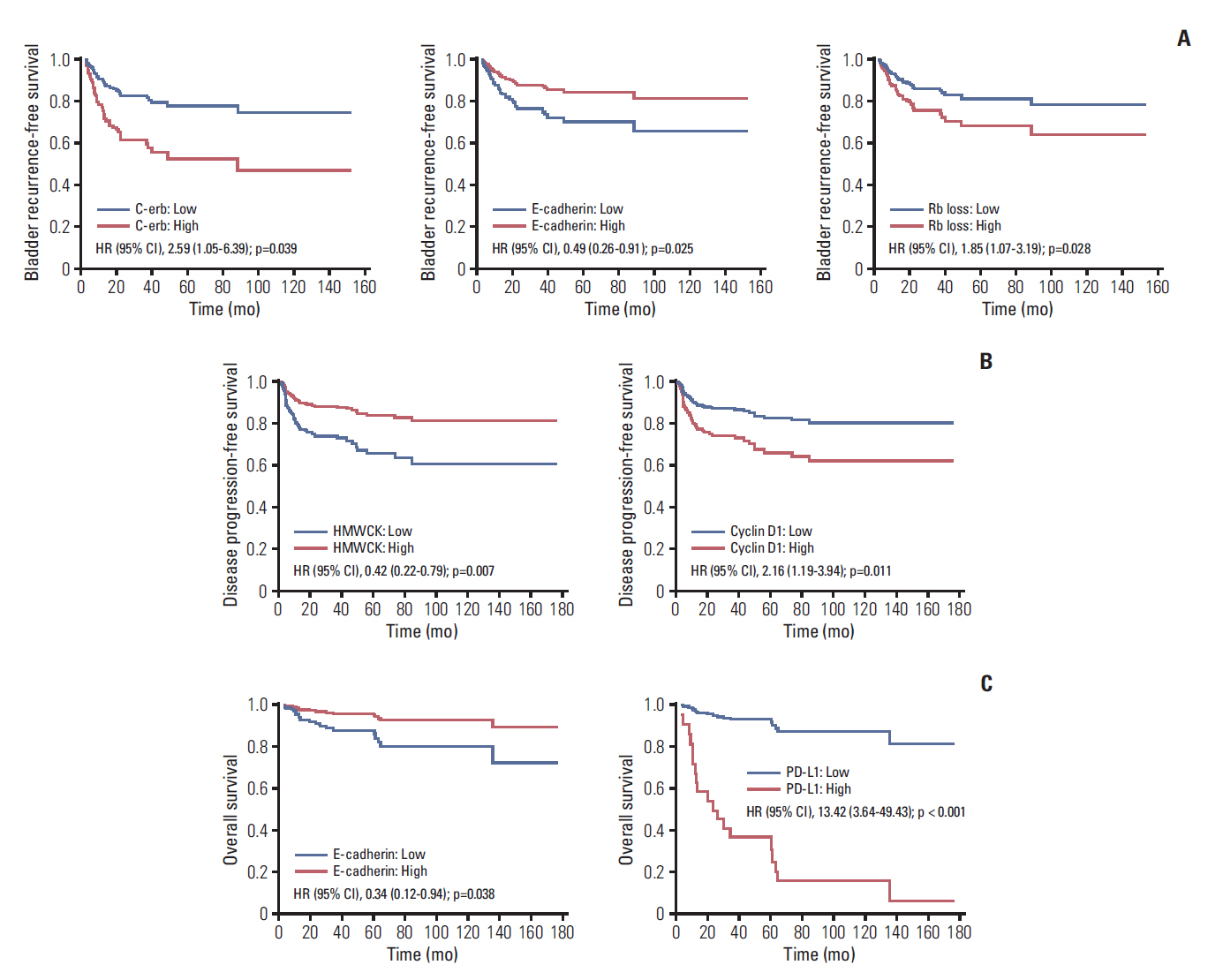
Median follow-up was 53.4 months (range, 3.6 to 176.5 months); and, 58 (35.8%), 48 (29.6%), and 19 (11.7%) bladder recurrence, disease progression, and all cause death, respectively, were identified. After adjusting for significant clinicopathological factors including intravesical instillation for bladder recurrence-free survival, pathologic T category and intravesical instillation for disease progression-free survival , and pathologic T category for OS (p < 0.05), IVRFS was associated with epithelial cadherin (hazard ratio [HR], 0.49), epidermal growth factor receptor/erythroblastosis oncogene B (c-erb) (HR, 2.59), and retinoblastoma protein loss (HR, 1.85); DFS was associated with cyclin D1 (HR, 2.16) and high-molecular-weight cytokeratin (HR, 0.42); OS was associated with E-cadherin (HR, 0.34) and programmed cell death 1 ligand (HR, 13.42) (p < 0.05).
Conclusion
Several significant tissue markers were associated with survival outcomes in upper urinary tract urothelial carcinoma patients treated with radical nephroureterectomy.
Keyword
Figure
Reference
-
References
1. Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009; 27:289–93.
Article2. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015; 68:868–79.3. Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol. 2007; 4:432–43.
Article4. Cha EK, Shariat SF, Kormaksson M, Novara G, Chromecki TF, Scherr DS, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2012; 61:818–25.
Article5. Yates DR, Hupertan V, Colin P, Ouzzane A, Descazeaud A, Long JA, et al. Cancer-specific survival after radical nephroureterectomy for upper urinary tract urothelial carcinoma: proposal and multi-institutional validation of a post-operative nomogram. Br J Cancer. 2012; 106:1083–8.
Article6. Chung PH, Krabbe LM, Darwish OM, Westerman ME, Bagrodia A, Gayed BA, et al. Degree of hydronephrosis predicts adverse pathological features and worse oncologic outcomes in patients with high-grade urothelial carcinoma of the upper urinary tract. Urol Oncol. 2014; 32:981–8.
Article7. Hwang I, Jung SI, Nam DH, Hwang EC, Kang TW, Kwon DD, et al. Preoperative hydronephrosis and diabetes mellitus predict poor prognosis in upper urinary tract urothelial carcinoma. Can Urol Assoc J. 2013; 7:E215–20.
Article8. Zhang X, Zhu Z, Zhong S, Xu T, Shen Z. Ureteral tumours showing a worse prognosis than renal pelvis tumours may be attributed to ureteral tumours more likely to have hydronephrosis and less likely to have haematuria. World J Urol. 2013; 31:155–60.
Article9. Rajcani J, Kajo K, Adamkov M, Moravekova E, Lauko L, Felcanova D, et al. Immunohistochemical characterization of urothelial carcinoma. Bratisl Lek Listy. 2013; 114:431–8.
Article10. Mazzucchelli R, Scarpelli M, Galosi AB, Di Primio R, Lopez-Beltran A, Cheng L, et al. Pathology of upper tract urothelial carcinoma with emphasis on staging. Int J Immunopathol Pharmacol. 2014; 27:509–16.
Article11. Xu S, Gu G, Ni Q, Li N, Yu K, Li X, et al. The expression of AEG-1 and cyclin D1 in human bladder urothelial carcinoma and their clinicopathological significance. Int J Clin Exp Med. 2015; 8:21222–8.12. Mann SA, Lopez-Beltran A, Massari F, Pili R, Fiorentino M, Koch MO, et al. Targeting the programmed cell death-1 pathway in genitourinary tumors: current progress and future perspectives. Curr Drug Metab. 2017; 18:700–11.
Article13. Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015; 26:812–7.
Article14. Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015; 106:945–50.
Article15. Reis ST, Leite KR, Mosconi Neto A, Pontes Junior J, Viana NI, Antunes AA, et al. Immune expression of E-cadherin and alpha, beta and gamma-catenin adhesion molecules and prognosis for upper urinary tract urothelial carcinomas. Int Braz J Urol. 2012; 38:466–73.16. Cho J, Ha SY, Kim SH, Sung HH, Kwon GY. Prognostic significance of epithelial-mesenchymal transition phenotypes in upper urinary tract urothelial carcinoma. Pathol Res Pract. 2018; 214:547–54.
Article17. Romkes M, Chern HD, Lesnick TG, Becich MJ, Persad R, Smith P, et al. Association of low CYP3A activity with p53 mutation and CYP2D6 activity with Rb mutation in human bladder cancer. Carcinogenesis. 1996; 17:1057–62.
Article18. Lonn U, Lonn S, Friberg S, Nilsson B, Silfversward C, Stenkvist B. Prognostic value of amplification of c-erb-B2 in bladder carcinoma. Clin Cancer Res. 1995; 1:1189–94.19. Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urol Oncol. 2010; 28:401–8.
Article20. Cordon-Cardo C. Molecular alterations associated with bladder cancer initiation and progression. Scand J Urol Nephrol Suppl. 2008; 154–65.
Article21. Korkolopoulou P, Christodoulou P, Kapralos P, Exarchakos M, Bisbiroula A, Hadjiyannakis M, et al. The role of p53, MDM2 and c-erb B-2 oncoproteins, epidermal growth factor receptor and proliferation markers in the prognosis of urinary bladder cancer. Pathol Res Pract. 1997; 193:767–75.
Article22. Vollmer RT, Humphrey PA, Swanson PE, Wick MR, Hudson ML. Invasion of the bladder by transitional cell carcinoma: its relation to histologic grade and expression of p53, MIB-1, c-erb B-2, epidermal growth factor receptor, and bcl-2. Cancer. 1998; 82:715–23.
Article23. Fontana LO, Garcia Garcia F, Arcas Martinez Salas I, Garcia Ligero J, Tomas Ros M, Rico Galiano JL, et al. The expression of p53 and c-erb-2 in transitional cell carcinoma of the kidney pelvis and ureter and its relation to tumor progression and survival. Arch Esp Urol. 2002; 55:792–6.24. Kunju LP, Mehra R, Snyder M, Shah RB. Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. Am J Clin Pathol. 2006; 125:675–81.25. Oya M, Schmidt B, Schmitz-Drager BJ, Schulz WA. Expression of G1-->S transition regulatory molecules in human urothelial cancer. Jpn J Cancer Res. 1998; 89:719–26.26. El-Gendi S, Abu-Sheasha G. Ki-67 and cell cycle regulators p53, p63 and cyclinD1 as prognostic markers for recurrence/progression of bladder urothelial carcinoma. Pathol Oncol Res. 2018; 24:309–22.27. Amin MB, Trpkov K, Lopez-Beltran A, Grignon D; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the bladder lesions: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014; 38:e20–34.28. Contal C, O’Quigley J. An application of checkpoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999; 30:253–70.29. Connolly SS, Rochester MA; BAUS. Nephroureterectomy surgery in the UK in 2012: British Association of Urological Surgeons (BAUS) Registry data. BJU Int. 2015; 116:780–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Urothelial Tumors of the Upper Urinary Tract: Multiplicity and Prognostic Variables
- Prognostic Factors in Transitional Cell Carcinoma of the Upper Urinary Tract after Radical Nephroureterectomy
- Prognostic Value of Lymphovascular Invasion in Node-Negative Upper Urinary Tract Urothelial Carcinoma Patients Undergoing Radical Nephroureterectomy
- Conditional Survival and Associated Prognostic Factors in Patients with Upper Tract Urothelial Carcinoma after Radical Nephroureterectomy: A Retrospective Study at a Single Institution
- Prognostic Factors for Survival in the Transitional Cell Carcinoma of the Upper Urinary Tract