Cancer Res Treat.
2020 Jan;52(1):10-23. 10.4143/crt.2019.145.
Cathepsin C Interacts with TNF-α/p38 MAPK Signaling Pathway to Promote Proliferation and Metastasis in Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Liver Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- KMID: 2501197
- DOI: http://doi.org/10.4143/crt.2019.145
Abstract
- Purpose
Although cathepsin C (CTSC) has been reported to maintain malignant biological properties in various cancers, its functions in hepatocellular carcinoma (HCC) remain obscure. We aimed to investigate the potential role of CTSC in HCC.
Materials and Methods
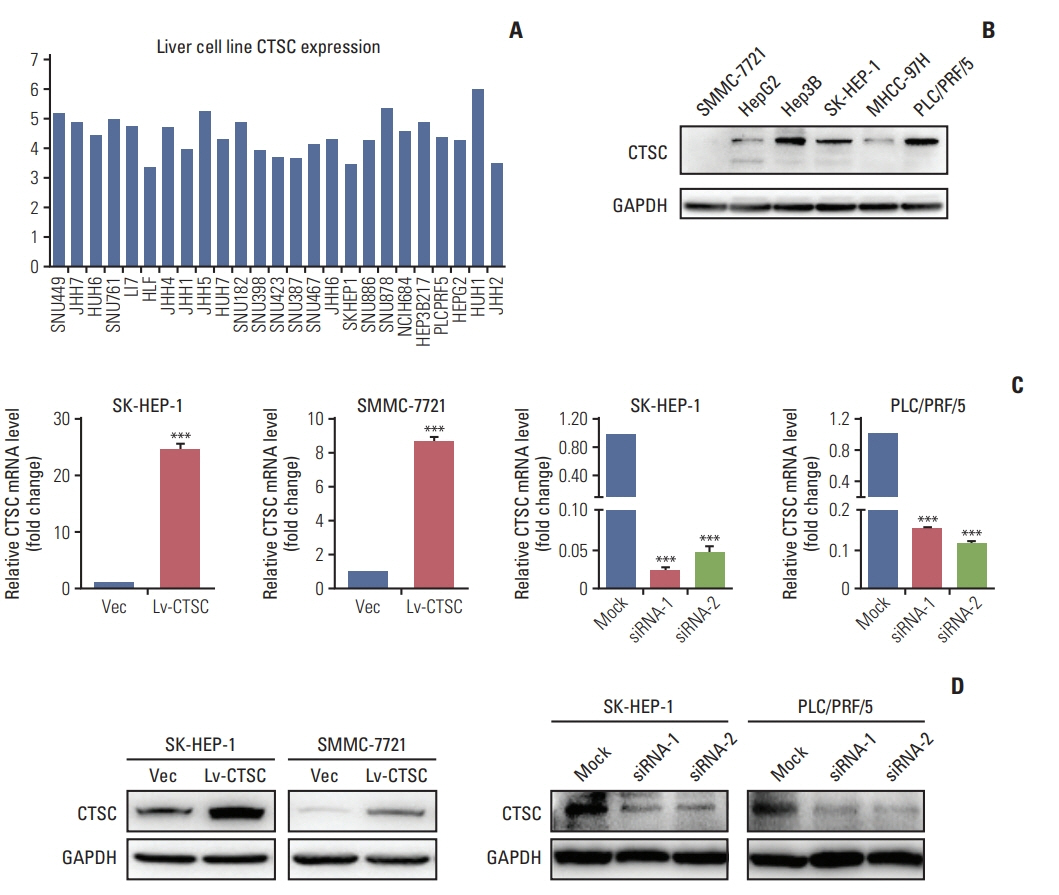
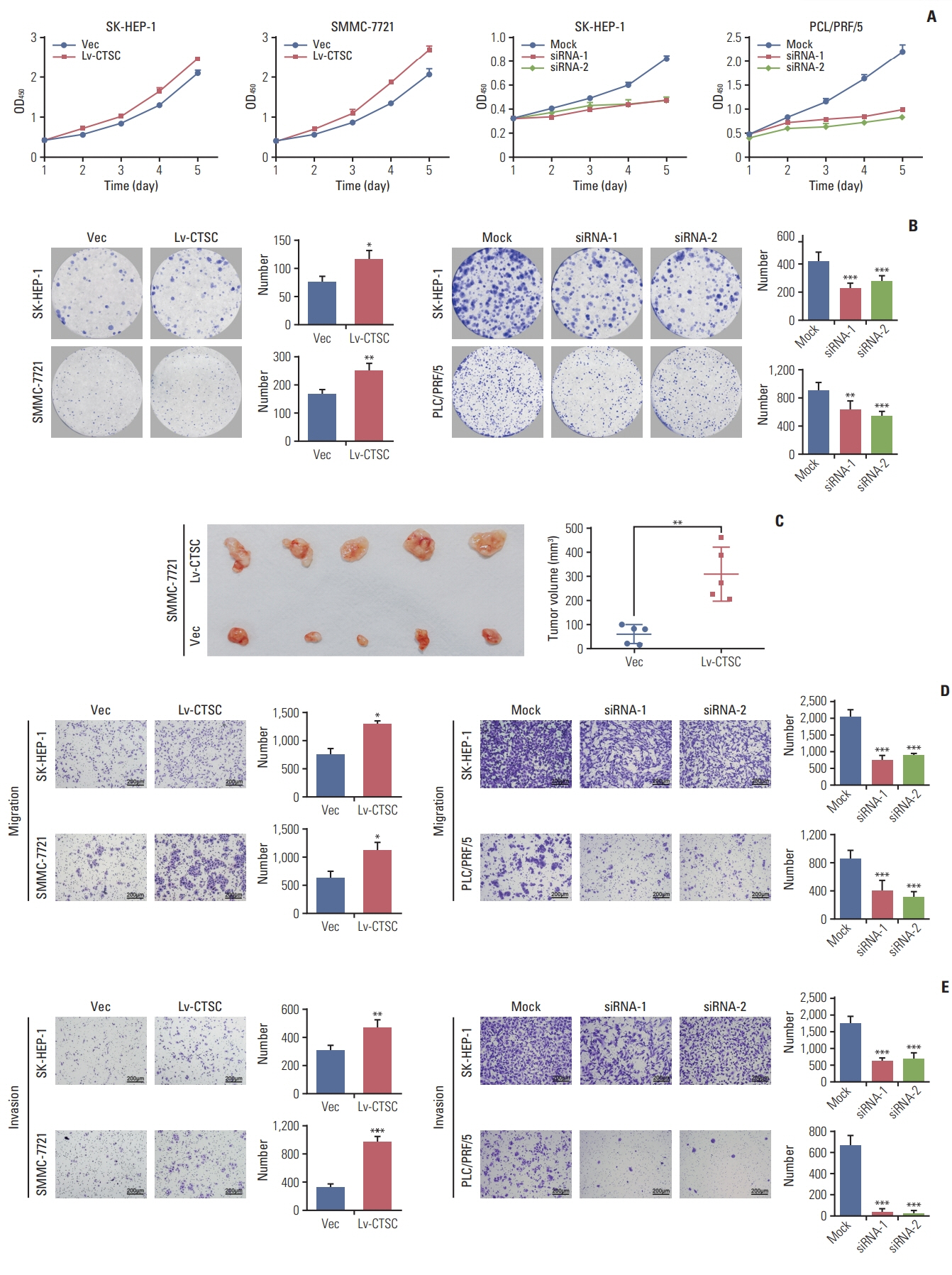
HCC tissue microarrays (n=122) were employed to analyze the correlation between CTSC expression and clinicopathological characteristics through immunohistochemistry staining. Quantitative real-time polymerase chain reaction, western blot assay, Cell Counting Kit-8 assay, colony formation, cell migration, and invasion assays, xenograft mice model were adopted to validate what had been indicated by the bioinformatic web tools.
Results
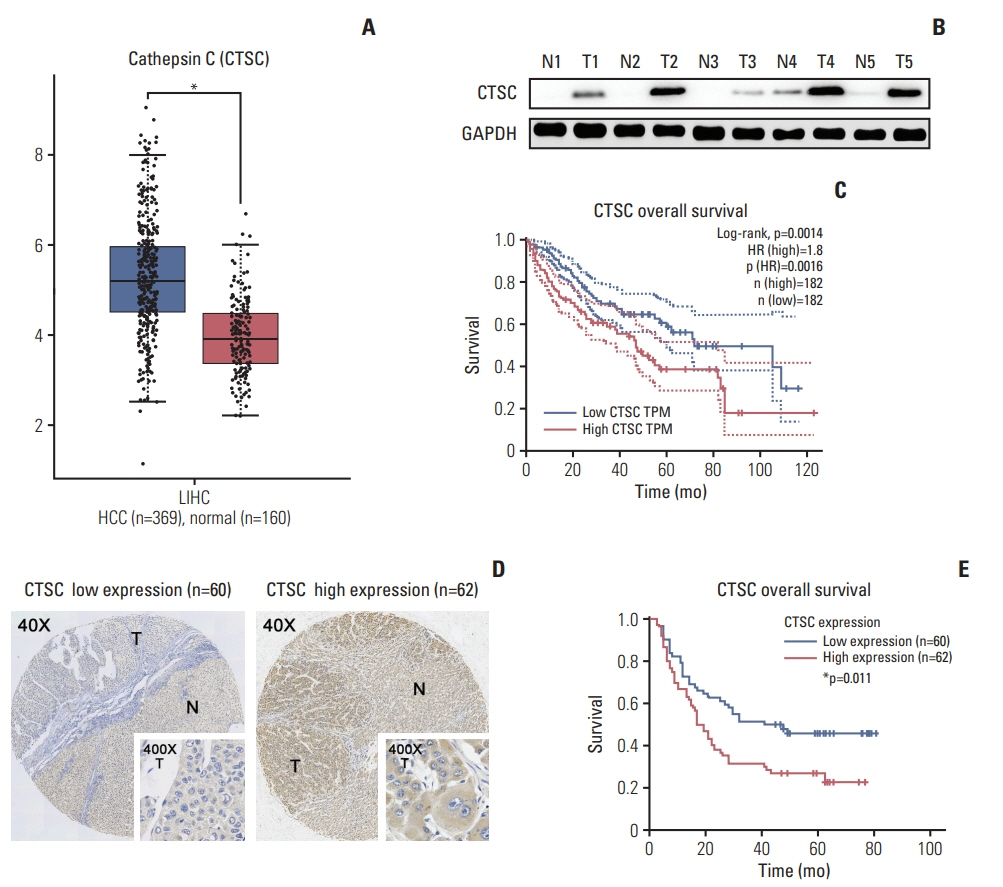
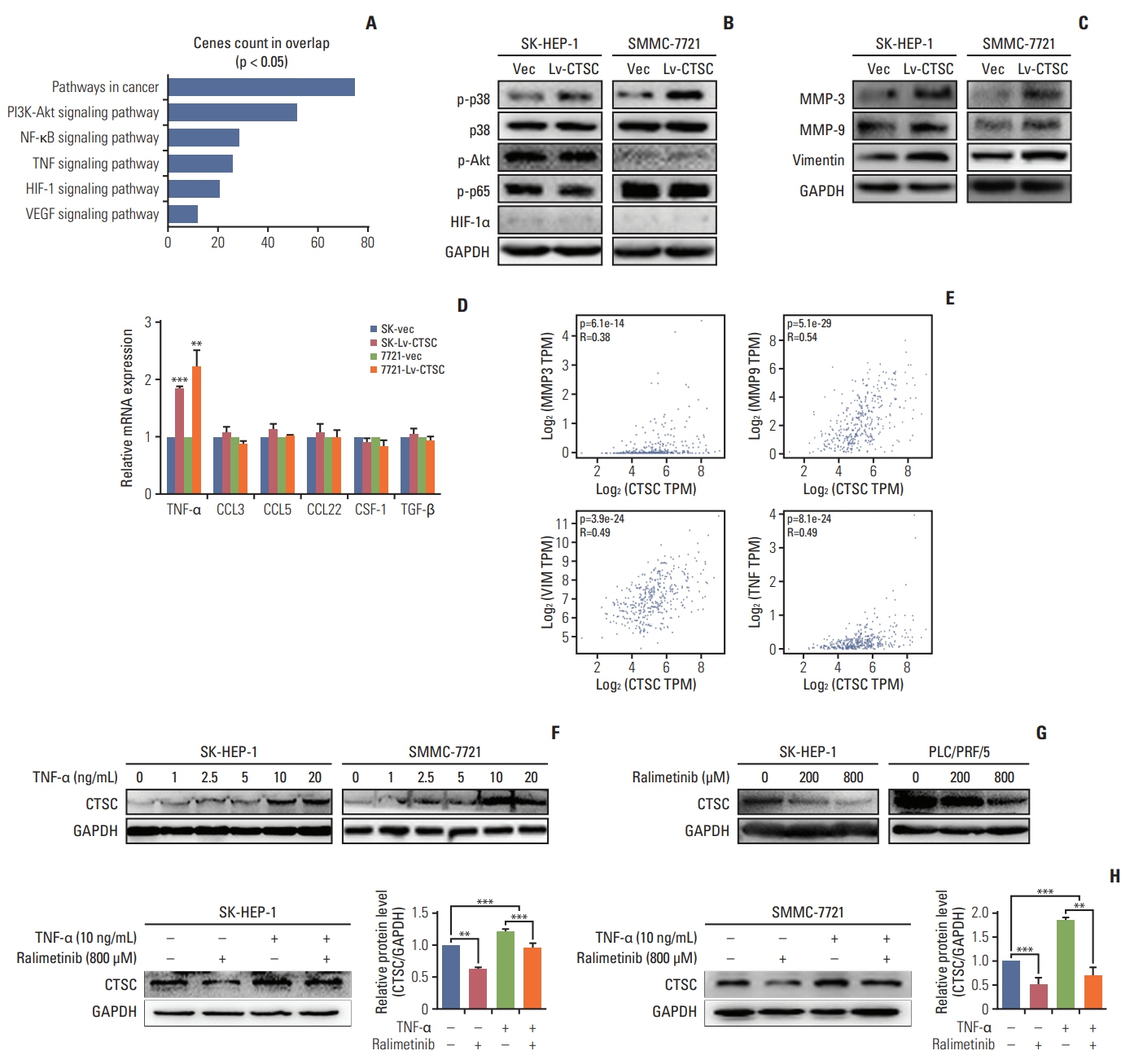
By bioinformatic tools and tissue microarrays, CTSC was found upregulated in HCC compared with normal liver tissues, and its higher expression was correlated with poor prognosis of HCC patients (hazard ratio, 2.402; 95% confidence interval, 1.493 to 3.865; p < 0.001). By gain/loss-of-function assays, we implicated that CTSC functioned as an oncogene to promote the proliferation and metastasis of HCC cells. Mechanistically, we revealed that CTSC was involved in several cancer-related signaling pathways by Gene Set Enrichment Analysis, among which tumor necrosis factor α (TNF-α)/p38 pathway was verified to be activated by CTSC. Furthermore, we found that TNF-α could activate CTSC expression in a concentration- dependent manner. Ralimetinib, an oral p38 mitogen-activated protein kinase (MAPK) inhibitor could inhibit CTSC expression. These indicated a potential positive feedback loop between CTSC and TNF-α/MAPK (p38) signaling.
Conclusion
Taken together, CTSC plays an important role in the growth and metastasis of HCC and may be a promising therapeutic target upon HCC.
Keyword
Figure
Reference
-
References
1. Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017; 67:302–9.
Article2. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015; 19:223–38.3. Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J Gastrointest Cancer. 2017; 48:238–40.
Article4. Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015; 9:765–79.
Article5. Yagi R, Midorikawa Y, Moriguchi M, Nakayama H, Aramaki O, Yamazaki S, et al. Liver resection for recurrent hepatocellular carcinoma to improve survivability: a proposal of indication criteria. Surgery. 2018; 163:1250–6.
Article6. Zhu J, Yin T, Xu Y, Lu XJ. Therapeutics for advanced hepatocellular carcinoma: Recent advances, current dilemma, and future directions. J Cell Physiol. 2019; 234:12122–32.
Article7. Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015; 15:712–29.
Article8. Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem. 2002; 383:1827–31.
Article9. Ruan J, Zheng H, Rong X, Rong X, Zhang J, Fang W, et al. Over-expression of cathepsin B in hepatocellular carcinomas predicts poor prognosis of HCC patients. Mol Cancer. 2016; 15:17.
Article10. Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, et al. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014; 60:179–91.
Article11. Ruffell B, Affara NI, Cottone L, Junankar S, Johansson M, DeNardo DG, et al. Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes Dev. 2013; 27:2086–98.
Article12. Joyce JA, Hanahan D. Multiple roles for cysteine cathepsins in cancer. Cell Cycle. 2004; 3:1516–619.
Article13. Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006; 20:543–56.
Article14. Khaket TP, Singh MP, Khan I, Bhardwaj M, Kang SC. Targeting of cathepsin C induces autophagic dysregulation that directs ER stress mediated cellular cytotoxicity in colorectal cancer cells. Cell Signal. 2018; 46:92–102.
Article15. Mikhaylov G, Mikac U, Magaeva AA, Itin VI, Naiden EP, Psakhye I, et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat Nanotechnol. 2011; 6:594–602.
Article16. Xia P, Zhang R, Ge G. C/EBPbeta mediates TNF-alphainduced cancer cell migration by inducing MMP expression dependent on p38 MAPK. J Cell Biochem. 2015; 116:2766–77.17. Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010; 140:197–208.
Article18. Jing Y, Sun K, Liu W, Sheng D, Zhao S, Gao L, et al. Tumor necrosis factor-alpha promotes hepatocellular carcinogenesis through the activation of hepatic progenitor cells. Cancer Lett. 2018; 434:22–32.19. Xu ZW, Yan SX, Wu HX, Chen JY, Zhang Y, Li Y, et al. The influence of TNF-alpha and Ang II on the proliferation, migration and invasion of HepG2 cells by regulating the expression of GRK2. Cancer Chemother Pharmacol. 2017; 79:747–58.20. Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, et al. Tumor cell-derived and macrophagederived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006; 66:5242–50.
Article21. Alapati K, Gopinath S, Malla RR, Dasari VR, Rao JS. uPAR and cathepsin B knockdown inhibits radiation-induced PKC integrated integrin signaling to the cytoskeleton of glioma-initiating cells. Int J Oncol. 2012; 41:599–610.
Article22. Li A, Shi D, Xu B, Wang J, Tang YL, Xiao W, et al. S100A6 promotes cell proliferation in human nasopharyngeal carcinoma via the p38/MAPK signaling pathway. Mol Carcinog. 2017; 56:972–84.
Article23. Urosevic J, Garcia-Albeniz X, Planet E, Real S, Cespedes MV, Guiu M, et al. Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nat Cell Biol. 2014; 16:685–94.24. Tang YM, Cao QY, Guo XY, Dong SH, Duan JA, Wu QN, et al. Inhibition of p38 and ERK1/2 pathways by Sparstolonin B suppresses inflammation-induced melanoma metastasis. Biomed Pharmacother. 2018; 98:382–9.
Article25. Bibikova E, Youn MY, Danilova N, Ono-Uruga Y, KontoGhiorghi Y, Ochoa R, et al. TNF-mediated inflammation represses GATA1 and activates p38 MAP kinase in RPS19-deficient hematopoietic progenitors. Blood. 2014; 124:3791–8.
Article26. Liu Q, Zhang Y, Liu S, Liu Y, Yang X, Liu G, et al. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca(2+)-dependent PKC/p38MAPK/NF-kappaB pathway. J Neuroinflammation. 2019; 16:10.
Article27. Jin X, Mo Q, Zhang Y, Gao Y, Wu Y, Li J, et al. The p38 MAPK inhibitor BIRB796 enhances the antitumor effects of VX680 in cervical cancer. Cancer Biol Ther. 2016; 17:566–76.
Article28. Guo X, Ma N, Wang J, Song J, Bu X, Cheng Y, et al. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer. 2008; 8:375.
Article29. Lim SJ, Lee YJ, Lee E. p38MAPK inhibitor SB203580 sensitizes human SNU-C4 colon cancer cells to exisulind-induced apoptosis. Oncol Rep. 2006; 16:1131–5.
Article30. Patnaik A, Haluska P, Tolcher AW, Erlichman C, Papadopoulos KP, Lensing JL, et al. A first-in-human phase I study of the oral p38 MAPK inhibitor, ralimetinib (LY2228820 Dimesylate), in patients with advanced cancer. Clin Cancer Res. 2016; 22:1095–102.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ras Mitogen-activated Protein Kinase Signaling and Kinase Suppressor of Ras as Therapeutic Targets for Hepatocellular Carcinoma
- Immunohistochemical Analysis of Nuclear Factor, p38, and Cyclin D1 Proteins in Premalignant Lesions and Carcinomas of the Colorectal Mucosa
- Nectandrin A Enhances the BMP-Induced Osteoblastic Differentiation and Mineralization by Activation of p38 MAPK-Smad Signaling Pathway
- HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways
- Synergistic Effect of Hydrogen and 5-Aza on Myogenic Differentiation through the p38 MAPK Signaling Pathway in Adipose-Derived Mesenchymal Stem Cells