J Endocr Surg.
2020 Mar;20(1):3-11. 10.16956/jes.2020.20.1.3.
Anaplastic Transformation of Metastatic Papillary Thyroid Carcinoma in a Cervical Lymph Node: a Timeline and Short Review
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Edith Wolfson Medical Center, Sackler School of Medicine, Tel Aviv University, Holon, Israel. udicin@yahoo.com
- 2Department of Pathology, Edith Wolfson Medical Center, Sackler School of Medicine, Tel Aviv University, Holon, Israel.
- KMID: 2471988
- DOI: http://doi.org/10.16956/jes.2020.20.1.3
Abstract
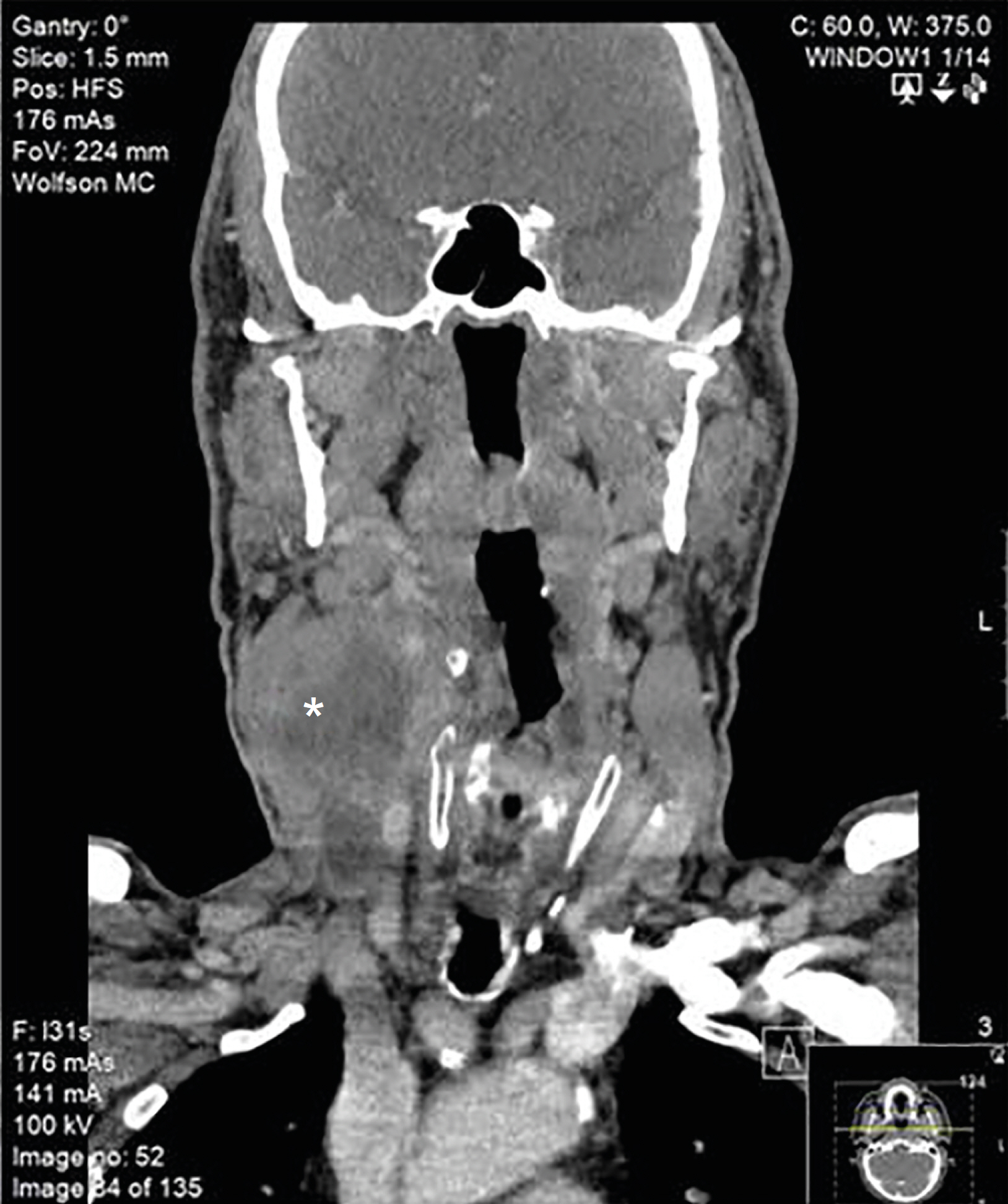
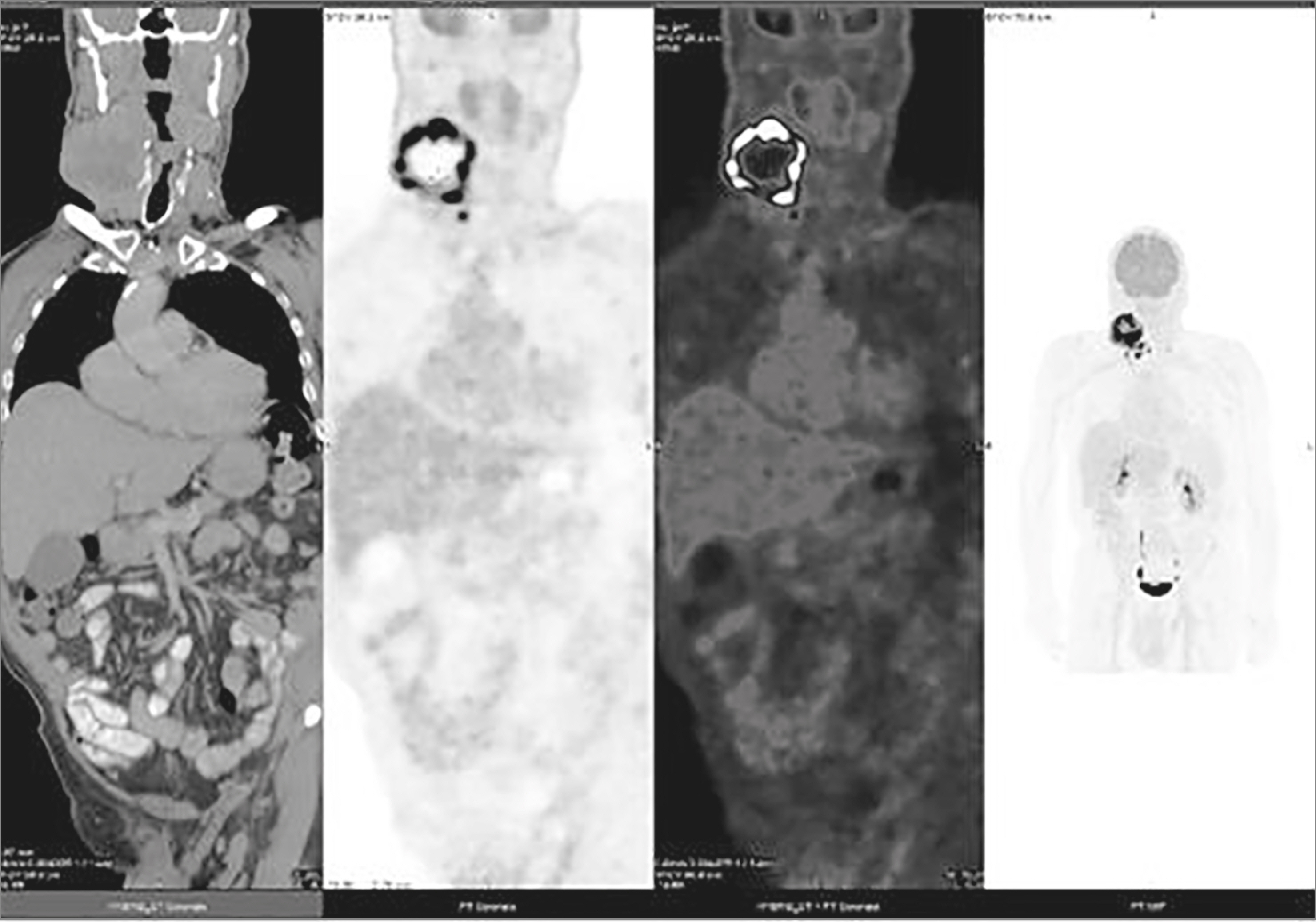
- Anaplastic thyroid cancer (ATC) is accepted as the transformation of a pre-existing glandular papillary thyroid carcinoma (PTC). Anaplastic transformation of a neck PTC metastasis in a lymph node is extraordinary. We present a patient with an exceptional timeline of an untreated neck PTC recurrence in a lymph node and its rare anaplastic transformation. A 68-year-old patient with PTC and neck metastasis, (stage III/II 7th/8th American Joint Committee on Cancer [AJCC], respectively) underwent thyroidectomy and neck dissection in July 2010, followed with radioiodine treatment (150 mCi) (August 2010). He received an additional 150 mCi in June 2012, because an iodine scan suggested right neck recurrence. In October 2013, an ultrasound revealed a 2.3 cm, suspicious right neck lymph node (level II-III). Yet only in 2017, after growing to 2.7 cm, the patient consented for a fine needle aspiration. PTC was verified, yet intervention was declined. In June 2018, he presented with a rapid growing neck mass occupying right levels II, III, carotid artery encasement and jugular vein involvement. A large bore needle biopsy revealed a highly malignant tumor, surrounded by necrosis, positive for cytokeratin (CK MNF 116), thyroid lineage marker (PAX8), negative for TTF-1 and thyroglobulin, i.e., ATC. The patient deceased in November 2018. This unique "natural history" of an untreated patient with PTC neck recurrence in a lymph node demonstrated a rare, yet a possible long-term consequence of anaplastic transformation. This case study, in addition to the sparsity of reported information, may advocate treating PTC neck recurrence.
MeSH Terms
-
Aged
Biopsy, Fine-Needle
Biopsy, Needle
Carotid Arteries
Cell Transformation, Neoplastic
Humans
Iodine
Joints
Jugular Veins
Keratins
Lymph Nodes*
Neck
Neck Dissection
Necrosis
Neoplasm Metastasis
Recurrence
Thyroglobulin
Thyroid Carcinoma, Anaplastic
Thyroid Gland*
Thyroid Neoplasms*
Thyroidectomy
Ultrasonography
Iodine
Keratins
Thyroglobulin
Figure
Reference
-
1.Patel KN., Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Contr. 2006. 13:119–28.
Article2.Wiseman SM., Loree TR., Rigual NR., Hicks WL Jr., Douglas WG., Anderson GR, et al. Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck. 2003. 25:662–70.
Article3.Oishi N., Kondo T., Ebina A., Sato Y., Akaishi J., Hino R, et al. Molecular alterations of coexisting thyroid papillary carcinoma and anaplastic carcinoma: identification of TERT mutation as an independent risk factor for transformation. Mod Pathol. 2017. 30:1527–37.
Article4.Ito Y., Higashiyama T., Hirokawa M., Fukushima M., Inoue H., Yabuta T, et al. Prognosis of patients with papillary carcinoma showing anaplastic transformation in regional lymph nodes that were curatively resected. Endocr J. 2008. 55:985–9.
Article5.Perrier ND., Brierley JD., Tuttle RM. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2018. 68:55–63.
Article6.Ragazzi M., Ciarrocchi A., Sancisi V., Gandolfi G., Bisagni A., Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014. 2014:790834.
Article7.Lo CY., Lam KY., Wan KY. Anaplastic carcinoma of the thyroid. Am J Surg. 1999. 177:337–9.
Article8.Togashi S., Oka K., Kanayama R., Koyamatsu S., Tobita T., Yatabe Y, et al. Thyroid anaplastic carcinoma transformed from papillary carcinoma in extrathyroid area. Auris Nasus Larynx. 2004. 31:287–92.
Article9.Bishop JA., Sharma R., Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011. 42:1873–7.
Article10.Kim H., Park YW., Oh YH., Sim J., Ro JY., Pyo JY. Anaplastic transformation of papillary thyroid carcinoma only seen in pleural metastasis: a case report with review of the literature. Head Neck Pathol. 2017. 11:162–7.
Article11.Sato K., Waseda R., Tatsuzawa Y., Soma R., Ueda Y., Katsuda S. Papillary thyroid carcinoma with anaplastic transformation showing a rhabdoid phenotype solely in the cervical lymph node metastasis. Pathol Res Pract. 2006. 202:55–9.
Article12.Benedict M., Costa J. Metastatic papillary thyroid carcinoma with multifocal synchronous transformation to anaplastic thyroid carcinoma. Case Rep Pathol. 2016. 2016:4863405.
Article13.Abe T., Suzuki M., Shimizu K., Shinagawa N., Oizumi S., Matsuno Y, et al. Anaplastic transformation of papillary thyroid carcinoma in multiple lung metastases presenting with a malignant pleural effusion: a case report. J Med Case Reports. 2014. 8:460.
Article14.Al-Qsous W., Miller ID. Anaplastic transformation in lung metastases of differentiated papillary thyroid carcinoma: an autopsy case report and review of the literature. Ann Diagn Pathol. 2010. 14:41–3.
Article15.Sotome K., Onishi T., Hirano A., Nakamaru M., Furukawa A., Miyazaki H, et al. A rare case of anaplastic transformation within the metastatic site of the retroperitoneal region in a patient 17 years after total thyroidectomy for papillary carcinoma of the thyroid beginning with multiple bone metastases. Thyroid. 2007. 17:1309–11.
Article16.Takeshita Y., Takamura T., Minato H., Misu H., Ando H., Yamashita T, et al. Transformation of p53-positive papillary thyroid carcinoma to anaplastic carcinoma of the liver following postoperative radioactive iodine-131 therapy. Intern Med. 2008. 47:1709–12.
Article17.Angeles-Angeles A., Chable-Montero F., Martinez-Benitez B., Albores-Saavedra J. Unusual metastases of papillary thyroid carcinoma: report of 2 cases. Ann Diagn Pathol. 2009. 13:189–96.
Article18.Kaushal S., Sharma MC., Mathur SR., Rastogi S., Bal CS., Chumber S. Anaplastic transformation of metastatic papillary thyroid carcinoma at shoulder mimicking soft tissue sarcoma. Indian J Pathol Microbiol. 2011. 54:796–9.19.Sugitani I., Miyauchi A., Sugino K., Okamoto T., Yoshida A., Suzuki S. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg. 2012. 36:1247–54.
Article20.Mansour J., Sagiv D., Alon E., Talmi Y. Prognostic value of lymph node ratio in metastatic papillary thyroid carcinoma. J Laryngol Otol. 2018. 132:8–13.
Article21.Shingu K., Kobayashi S., Yokoyama S., Fujimori M., Asanuma K., Ito KI, et al. The likely transformation of papillary thyroid carcinoma into anaplastic carcinoma during postoperative radioactive iodine-131 therapy: report of a case. Surg Today. 2000. 30:910–3.
Article22.Sera N., Ashizawa K., Ando T., Ide A., Abe Y., Usa T, et al. Anaplastic changes associated with p53 gene mutation in differentiated thyroid carcinoma after insufficient radioactive iodine (131 I) therapy. Thyroid. 2000. 10:975–9.23.Manzella L., Stella S., Pennisi MS., Tirrò E., Massimino M., Romano C, et al. New insights in thyroid cancer and p53 family proteins. Int J Mol Sci. 2017. 18:1325.
Article24.Quiros RM., Ding HG., Gattuso P., Prinz RA., Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005. 103:2261–8.25.Hershman JM., Okunyan A., Rivina Y., Cannon S., Hogen V. Prevention of DNA double-strand breaks induced by radioiodide-131 I in FRTL-5 thyroid cells. Endocrinology. 2011. 152:1130–5.26.Turgut B., Ozdemir O., Erselcan T. Evaluation of the p53 tumor suppressor gene mutation in normal rat salivary gland tissue after radioiodine application: an experimental study. Adv Ther. 2006. 23:456–68.27.Gamble SC., Cook MC., Riches AC., Herceg Z., Bryant PE., Arrand JE. p53 mutations in tumors derived from irradiated human thyroid epithelial cells. Mutat Res. 1999. 425:231–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anaplastic Transformation of Metastatic Papillary Thyroid Carcinomas in the Cervical Lymph Nodes: Report of 3 Cases
- Coexistent Papillary Thyroid Carcinoma and Its Anaplastic Transformation in Cervical Lymph Node Metastasis
- Anaplastic Transformation of Follicular Thyroid Cancer in the Lung, Liver, Bone, and Adrenal Gland
- Co-existence of Papillary Thyroid Cancer with Tuberculosis Involving the Thyroid and Ipsilateral Paratracheal Lymph Node: A Case Report
- A Case of Cystic Lymph Node Metastasis from Thyroid Papillary Microcarcinoma