Korean J Radiol.
2020 Apr;21(4):471-482. 10.3348/kjr.2019.0839.
Optimized Image-Based Surrogate Endpoints in Targeted Therapies for Glioblastoma: A Systematic Review and Meta-Analysis of Phase III Randomized Controlled Trials
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. radhskim@gmail.com
- KMID: 2471813
- DOI: http://doi.org/10.3348/kjr.2019.0839
Abstract
OBJECTIVE
We aimed to determine the optimized image-based surrogate endpoints (IBSEs) in targeted therapies for glioblastoma through a systematic review and meta-analysis of phase III randomized controlled trials (RCTs).
MATERIALS AND METHODS
A systematic search of OVID-MEDLINE and EMBASE for phase III RCTs on glioblastoma was performed in December 2017. Data on overall survival (OS) and IBSEs, including progression-free survival (PFS), 6-month PFS (6moPFS), 12-month PFS (12moPFS), median PFS, and objective response rate (ORR) were extracted. Weighted linear regression analysis for the hazard ratio for OS and the hazard ratios or odds ratios for IBSEs was performed. The associations between IBSEs and OS were evaluated. Subgroup analyses according to disease stage (newly diagnosed glioblastoma versus recurrent glioblastoma), types of test treatment, and types of response assessment criteria were performed.
RESULTS
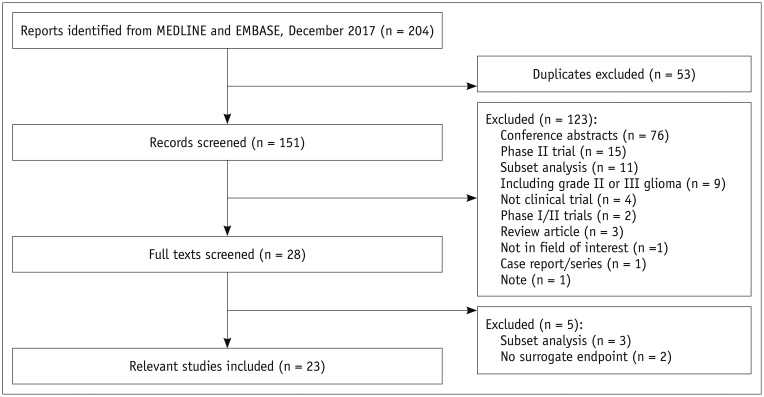
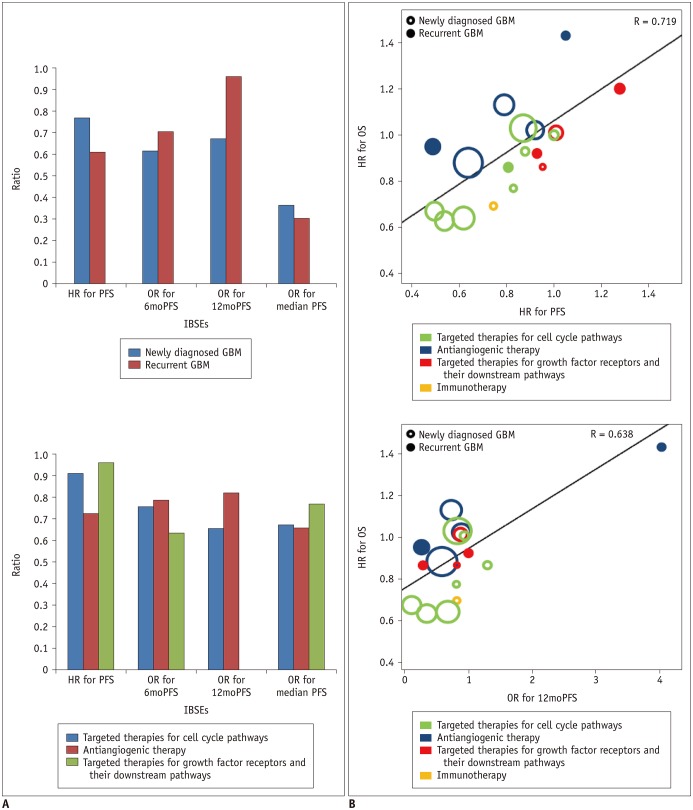
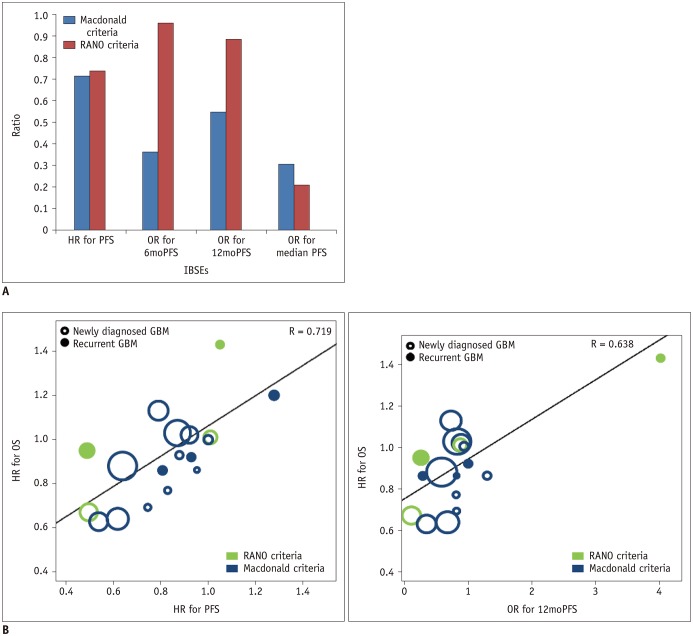
Twenty-three phase III RCTs published between 2000 and 2017, including 8387 patients, met the inclusion criteria. OS showed strong correlations with PFS (standardized β coefficient [R] = 0.719), 6moPFS (R = 0.647), and 12moPFS (R = 0.638). OS showed no correlations with median PFS and ORR. In subgroup analysis according to types of therapies, PFS showed the highest correlations with OS in targeted therapies for cell cycle pathways (R = 0.913) and growth factor receptors and their downstream pathways (R = 0.962). 12moPFS showed the highest correlation with OS in antiangiogenic therapy (R = 0.821). The response assessment in neuro-oncology criteria provided higher correlation coefficients between OS and IBSEs than the Macdonald criteria.
CONCLUSION
Overall, PFS is an optimized IBSE in targeted therapies for glioblastoma; however, 12moPFS is optimal in antiangiogenic therapy.
MeSH Terms
Figure
Reference
-
1. Barchana M, Margaliot M, Liphshitz I. Changes in brain glioma incidence and laterality correlates with use of mobile phones--a nationwide population based study in Israel. Asian Pac J Cancer Prev. 2012; 13:5857–5863. PMID: 23317269.2. Martell RE, Sermer D, Getz K, Kaitin KI. Oncology drug development and approval of systemic anticancer therapy by the U.S. Food and Drug Administration. Oncologist. 2013; 18:104–111. PMID: 23263289.
Article3. Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013; 31:3212–3218. PMID: 23940216.
Article4. Blumenthal DT, Rankin C, Stelzer KJ, Spence AM, Sloan AE, Moore DF Jr, et al. A Phase III study of radiation therapy (RT) and O6-benzylguanine + BCNU versus RT and BCNU alone and methylation status in newly diagnosed glioblastoma and gliosarcoma: Southwest Oncology Group (SWOG) study S0001. Int J Clin Oncol. 2015; 20:650–658. PMID: 25407559.
Article5. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014; 370:709–722. PMID: 24552318.
Article6. Dresemann G, Weller M, Rosenthal MA, Wedding U, Wagner W, Engel E, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010; 96:393–402. PMID: 19688297.
Article7. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014; 370:699–708. PMID: 24552317.
Article8. Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013; 31:4085–4091. PMID: 24101040.
Article9. Kim IH, Park CK, Heo DS, Kim CY, Rhee CH, Nam DH, et al. Radiotherapy followed by adjuvant temozolomide with or without neoadjuvant ACNU-CDDP chemotherapy in newly diagnosed glioblastomas: a prospective randomized controlled multicenter phase III trial. J Neurooncol. 2011; 103:595–602. PMID: 21052775.10. Kong DS, Nam DH, Kang SH, Lee JW, Chang JH, Kim JH, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017; 8:7003–7013. PMID: 27690294.
Article11. Levin VA, Uhm JH, Jaeckle KA, Choucair A, Flynn PJ, Yung WKA, et al. Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res. 2000; 6:3878–3884. PMID: 11051233.12. Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012; 13:916–926. PMID: 22877848.
Article13. Perry JR, Laperriere N, O'Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017; 376:1027–1037. PMID: 28296618.
Article14. Prados MD, Wara WM, Sneed PK, McDermott M, Chang SM, Rabbitt J, et al. Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001; 49:71–77. PMID: 11163499.
Article15. Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000; 11:2389–2401. PMID: 11096443.16. Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D, et al. International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015; 33:4145–4150. PMID: 26392096.
Article17. Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15:1100–1108. PMID: 25163906.
Article18. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.
Article19. Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015; 314:2535–2543. PMID: 26670971.20. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012; 48:2192–2202. PMID: 22608262.
Article21. Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017; 18:1373–1385. PMID: 28844499.22. Westphal M, Heese O, Steinbach JP, Schnell O, Schackert G, Mehdorn M, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015; 51:522–532. PMID: 25616647.
Article23. Westphal M, Ylä-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013; 14:823–833. PMID: 23850491.
Article24. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017; 377:1954–1963. PMID: 29141164.
Article25. Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010; 28:1168–1174. PMID: 20124186.
Article26. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009; 22:633–638. PMID: 19770760.
Article27. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017; 35:2439–2449. PMID: 28640707.
Article28. Zhao F. Surrogate end points and their validation in oncology clinical trials. J Clin Oncol. 2016; 34:1436–1437. PMID: 26951314.
Article29. Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017; 28:1457–1472. PMID: 28863449.
Article30. Burnand B, Kernan WN, Feinstein AR. Indexes and boundaries for “quantitative significance” in statistical decisions. J Clin Epidemiol. 1990; 43:1273–1284. PMID: 2254764.
Article31. Armstrong TS, Wefel JS, Wang M, Gilbert MR, Won M, Bottomley A, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013; 31:4076–4084. PMID: 24101048.
Article32. Mirimanoff RO, Gorlia T, Mason W, Van den Bent MJ, Kortmann RD, Fisher B, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006; 24:2563–2569. PMID: 16735709.
Article33. Taphoorn MJ, Henriksson R, Bottomley A, Cloughesy T, Wick W, Mason WP, et al. Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015; 33:2166–2175. PMID: 26014298.
Article34. Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010; 12:871–881. PMID: 20511192.
Article35. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466. PMID: 19269895.
Article36. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280. PMID: 2358840.
Article37. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28:1963–1972. PMID: 20231676.
Article38. Zer A, Prince RM, Amir E, Abdul Razak A. Evolution of randomized trials in advanced/metastatic soft tissue sarcoma: end point selection, surrogacy, and quality of reporting. J Clin Oncol. 2016; 34:1469–1475. PMID: 26951308.
Article39. Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008; 26:5458–5464. PMID: 18955452.
Article40. Foster NR, Renfro LA, Schild SE, Redman MW, Wang XF, Dahlberg SE, et al. Multitrial evaluation of progression-free survival as a surrogate end point for overall survival in first-line extensive-stage small-cell lung cancer. J Thorac Oncol. 2015; 10:1099–1106. PMID: 26134227.
Article41. Burzykowski T, Buyse M, Piccart-Gebhart MJ, Sledge G, Carmichael J, Lück HJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008; 26:1987–1992. PMID: 18421050.
Article42. Ballman KV, Buckner JC, Brown PD, Giannini C, Flynn PJ, LaPlant BR, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007; 9:29–38. PMID: 17108063.
Article43. Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008; 10:162–170. PMID: 18356283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Introduction to systematic review and meta-analysis
- Surrogate Endpoints in Second-Line Trials of Targeted Agents in Metastatic Colorectal Cancer: A Literature-Based Systematic Review and Meta-Analysis
- Effect of intracanal cryotherapy on postendodontic pain: a systematic review and meta-analysis of randomized controlled trials
- Website and Mobile Application-Based Interventions for Adolescents and Young Adults with Depression: A Systematic Review and Meta-Analysis
- Efficacy and safety of tofacitinib for active rheumatoid arthritis with an inadequate response to methotrexate or disease-modifying antirheumatic drugs: a meta-analysis of randomized controlled trials