Diabetes Metab J.
2020 Feb;44(1):134-142. 10.4093/dmj.2018.0273.
Plasma CD36 and Incident Diabetes: A Case-Cohort Study in Danish Men and Women
- Affiliations
-
- 1Health Services and Systems Research, Duke-NUS Medical School, Singapore.
- 2Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA. mkjensen@hsph.harvard.edu
- 3Department of Cardiology, Aalborg University Hospital, Aalborg, Denmark.
- 4Section for Epidemiology, Department of Public Health, Aarhus University, Aarhus, Denmark.
- 5Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
- 6Danish Cancer Society Research Center, Copenhagen, Denmark.
- 7Department of Clinical Biochemistry, Aalborg University Hospital, Aalborg, Denmark.
- 8Department of Clinical Medicine, Faculty of Medicine, Aalborg University, Aalborg, Denmark.
- 9Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
- KMID: 2470962
- DOI: http://doi.org/10.4093/dmj.2018.0273
Abstract
- BACKGROUND
Membrane CD36 is a fatty acid transporter implicated in the pathogenesis of metabolic disease. We aimed to evaluate the association between plasma CD36 levels and diabetes risk and to examine if the association was independent of adiposity among Danish population.
METHODS
We conducted a case-cohort study nested within the Danish Diet, Cancer and Health study among participants free of cardiovascular disease, diabetes and cancer and with blood samples and anthropometric measurements (height, weight, waist circumference, and body fat percentage) at baseline (1993 to 1997). CD36 levels were measured in 647 incident diabetes cases that occurred before December 2011 and a total of 3,515 case-cohort participants (236 cases overlap).
RESULTS
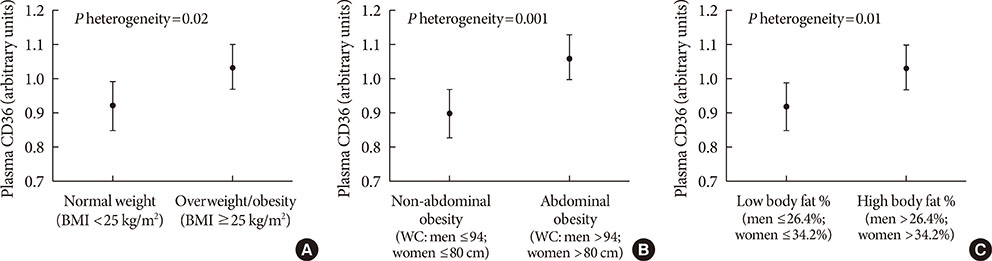
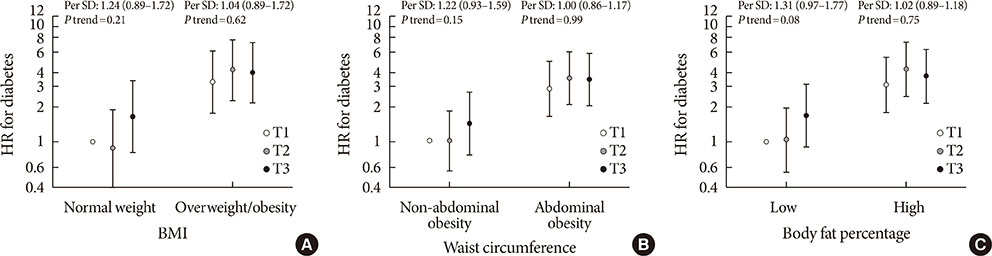
Higher plasma CD36 levels were associated with higher diabetes risk after adjusting for age, sex and other lifestyle factors. The hazard ratio (HR) comparing high versus low tertile of plasma CD36 levels was 1.36 (95% confidence interval [CI], 1.00 to 1.86). However, the association lost its significance after further adjustment for different adiposity indices such as body mass index (HR, 1.23; 95% CI, 0.87 to 1.73), waist circumference (HR, 1.21; 95% CI, 0.88 to 1.68) or body fat percentage (HR, 1.20; 95% CI, 0.86 to 1.66). Moreover, raised plasma CD36 levels were moderately associated with diabetes risk among lean participants, but the association was not present among overweight/obese individuals.
CONCLUSION
Higher plasma CD36 levels were associated with higher diabetes risk, but the association was not independent of adiposity. In this Danish population, the association of CD36 with diabetes risk could be either mediated or confounded by adiposity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
Diabetes Metab J. 2020;44(2):222-233. doi: 10.4093/dmj.2020.0053.
Reference
-
1. World Health Organization: Global Report on Diabetes. cited 2019 Jul 10. Available from: http://www.who.int/diabetes/global-report/en/.2. Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, Smith RJ, Smith SR. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011; 96:1654–1663.
Article3. Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010; 90:367–417.
Article4. Snel M, Jonker JT, Schoones J, Lamb H, de Roos A, Pijl H, Smit JW, Meinders AE, Jazet IM. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. 2012; 2012:983814.
Article5. An P, Freedman BI, Hanis CL, Chen YD, Weder AB, Schork NJ, Boerwinkle E, Province MA, Hsiung CA, Wu X, Quertermous T, Rao DC. Genome-wide linkage scans for fasting glucose, insulin, and insulin resistance in the National Heart, Lung, and Blood Institute Family Blood Pressure Program: evidence of linkages to chromosome 7q36 and 19q13 from meta-analysis. Diabetes. 2005; 54:909–914.
Article6. Arya R, Blangero J, Williams K, Almasy L, Dyer TD, Leach RJ, O'Connell P, Stern MP, Duggirala R. Factors of insulin resistance syndrome: related phenotypes are linked to genetic locations on chromosomes 6 and 7 in nondiabetic Mexican-Americans. Diabetes. 2002; 51:841–847.7. Malhotra A, Elbein SC, Ng MC, Duggirala R, Arya R, Imperatore G, Adeyemo A, Pollin TI, Hsueh WC, Chan JC, Rotimi C, Hanson RL, Hasstedt SJ, Wolford JK. Meta-analysis of genome-wide linkage studies of quantitative lipid traits in families ascertained for type 2 diabetes. Diabetes. 2007; 56:890–896.
Article8. Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004; 18:1144–1146.
Article9. Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004; 113:764–773.
Article10. Sampson MJ, Davies IR, Braschi S, Ivory K, Hughes DA. Increased expression of a scavenger receptor (CD36) in monocytes from subjects with type 2 diabetes. Atherosclerosis. 2003; 167:129–134.
Article11. Handberg A, Levin K, Hojlund K, Beck-Nielsen H. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation. 2006; 114:1169–1176.12. Heeboll S, Poulsen MK, Ornstrup MJ, Kjaer TN, Pedersen SB, Nielsen S, Gronbaek H, Handberg A. Circulating sCD36 levels in patients with non-alcoholic fatty liver disease and controls. Int J Obes (Lond). 2017; 41:262–267.13. Glintborg D, Hojlund K, Andersen M, Henriksen JE, Beck-Nielsen H, Handberg A. Soluble CD36 and risk markers of insulin resistance and atherosclerosis are elevated in polycystic ovary syndrome and significantly reduced during pioglitazone treatment. Diabetes Care. 2008; 31:328–334.
Article14. Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, Eriksson JW. Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab. 2010; 95:1939–1946.
Article15. Tjonneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, Overvad K. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007; 35:432–441.
Article16. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999; 52:1165–1172.
Article17. Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008; 167:653–666.
Article18. World Health Organization: Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. cited 2019 Jul 10. Available from: http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf.19. Stegger JG, Schmidt EB, Obel T, Berentzen TL, Tjonneland A, Sorensen TI, Overvad K. Body composition and body fat distribution in relation to later risk of acute myocardial infarction: a Danish follow-up study. Int J Obes (Lond). 2011; 35:1433–1441.
Article20. Payne R, Neykov M, Jensen MK, Cai T. Kernel machine testing for risk prediction with stratified case cohort studies. Biometrics. 2016; 72:372–381.
Article21. Samovski D, Dhule P, Pietka T, Jacome-Sosa M, Penrose E, Son NH, Flynn CR, Shoghi KI, Hyrc KL, Goldberg IJ, Gamazon ER, Abumrad NA. Regulation of insulin receptor pathway and glucose metabolism by CD36 signaling. Diabetes. 2018; 67:1272–1284.
Article22. Khan S, Kowluru A. CD36 mediates lipid accumulation in pancreatic beta cells under the duress of glucolipotoxic conditions: novel roles of lysine deacetylases. Biochem Biophys Res Commun. 2018; 495:2221–2226.
Article23. Elumalai S, Karunakaran U, Lee IK, Moon JS, Won KC. Rac1-NADPH oxidase signaling promotes CD36 activation under glucotoxic conditions in pancreatic beta cells. Redox Biol. 2017; 11:126–134.
Article24. Asakawa H, Tokunaga K, Kawakami F. Relationship of abdominal fat with metabolic disorders in diabetes mellitus patients. Diabetes Res Clin Pract. 2002; 55:139–149.
Article25. Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000; 278:E941–E948.26. Zhang D, Zhang R, Liu Y, Sun X, Yin Z, Li H, Zhao Y, Wang B, Ren Y, Cheng C, Liu X, Liu D, Liu F, Chen X, Liu L, Zhou Q, Xiong Y, Xu Q, Liu J, Hong S, You Z, Hu D, Zhang M. CD36 gene variants is associated with type 2 diabetes mellitus through the interaction of obesity in rural Chinese adults. Gene. 2018; 659:155–159.
Article27. Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999; 21:76–83.
Article28. Koonen DP, Jensen MK, Handberg A. Soluble CD36- a marker of the (pathophysiological) role of CD36 in the metabolic syndrome? Arch Physiol Biochem. 2011; 117:57–63.
Article29. American Heart Association: About Metabolic Syndrome. cited 2019 Jul 10. Available from: https://www.heart.org/HEARTORG/Conditions/More/MetabolicSyndrome/About-Metabolic-Syndrome_UCM_301920_Article.jsp.30. Norberg M, Stenlund H, Lindahl B, Andersson C, Weinehall L, Hallmans G, Eriksson JW. Components of metabolic syndrome predicting diabetes: no role of inflammation or dyslipidemia. Obesity. 2007; 15:1875–1885.
Article31. Griffin E, Re A, Hamel N, Fu C, Bush H, McCaffrey T, Asch AS. A link between diabetes and atherosclerosis: glucose regulates expression of CD36 at the level of translation. Nat Med. 2001; 7:840–846.
Article32. Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond). 2006; 30:877–883.
Article33. Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002; 277:49982–49988.
Article34. Love-Gregory L, Sherva R, Schappe T, Qi JS, McCrea J, Klein S, Connelly MA, Abumrad NA. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum Mol Genet. 2011; 20:193–201.
Article35. Love-Gregory L, Sherva R, Sun L, Wasson J, Schappe T, Doria A, Rao DC, Hunt SC, Klein S, Neuman RJ, Permutt MA, Abumrad NA. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet. 2008; 17:1695–1704.
Article36. Thunander M, Petersson C, Jonzon K, Fornander J, Ossiansson B, Torn C, Edvardsson S, Landin-Olsson M. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008; 82:247–255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Uterine Leiomyoma with Type 2 Diabetes Mellitus in Young Women: A Population-Based Cohort Study
- Iron Overload and the Risk of Diabetes in the General Population: Results of the Chinese Health and Nutrition Survey Cohort Study
- Egg consumption is associated with a lower risk of type 2 diabetes in middle-aged and older men
- The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
- Sex-specific associations between dietary legume subtypes and type 2 diabetes in a prospective cohort study